Abstract
We present a 58-year-old woman who presented with a 1.5-cm, hypodense lesion in the head of the pancreas. Endoscopic ultrasound-guided fine-needle aspiration yielded bland, monotonous cells with wispy cytoplasm, slightly granular chromatin, and small nucleoli. A presumptive diagnosis of a neuroendocrine lesion was rendered. Whipple procedure yielded a well-circumscribed, encapsulated lesion with dense, hyalinized stroma and a peripheral rim of lymphocytes. Spindled and epithelioid cells formed short tubules, cords, and nests. The neoplasm stained for CK 5/6, calretinin, vimentin, CD 99, pancytokeratin, and EMA, consistent with mesothelial origin. This characteristic histology and immunohistochemistry is consistent with an adenomatoid tumor. We believe we are the first to report this benign neoplasm in such an unusual location. Herein we address the diagnosis of adenomatoid tumor by histology, immunohistochemistry, and aspiration cytology. Our case is particularly unique in that the histology and cytology are compared and correlated.
Similar content being viewed by others
INTRODUCTION
Adenomatoid tumors are benign mesothelial neoplasms, most commonly encountered in the male and female reproductive system. The most common neoplasm of the epididymis (1), adenomatoid tumors are also frequently seen in the spermatic cord, tunica albuginea, prostate, and ejaculatory duct (2, 3). In the female genital tract, these lesions are encountered in the fallopian tube, uterus, and ovarian hilum (2). Sporadic case reports have also noted adenomatoid tumors in such varied extragenital locations as the adrenal gland (4, 5), small intestines, omentum, retroperitoneum, bladder, and pleura (3, 4, 5). To date, both ultrastructural and immunohistochemical evidence have demonstrated this tumor’s mesothelial origin (2, 3, 5). In this context, we present an adenomatoid tumor of the pancreas. To our knowledge, this is the first reported case in this location. Herein we report both cytological and histochemical results documenting this benign neoplasm in an unusual location.
CASE REPORT
A 58-year-old woman with a history of hypertension, cholecystitis, and arthritis presented with a mildly elevated alkaline phosphatase level. CT scan revealed a well-circumscribed, hypodense, 1.7-cm mass in the head of the pancreas. No additional lesions were noted in the chest, abdomen, or pelvis. An endoscopic ultrasound-guided fine-needle aspiration biopsy yielded cells consistent with a neuroendocrine neoplasm (Figs. 1, 2). However, the tumor was noted to be of low attenuation and vascularity, more consistent with an adenocarcinoma than a neuroendocrine tumor. The octreotide scan used to localize a neuroendocrine neoplasm was negative. The patient underwent an uneventful Whipple procedure.
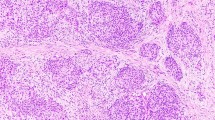
A–H, cytological and histological features of adenomatoid tumors. A, Diff Quik stain reveals a fairly cellular aspirate with round to ovoid, monotonous loosely aggregated cell clusters (DQ stain; 400×). B, the finely granular and evenly dispersed chromatin is shown under oil magnification (Pap stain; 1000×). C–D, side-by-side comparison of cytology and histology shows good correlation (Pap stain, 400×; hematoxylin-eosin, 400×). E, low-power photomicrograph demonstrates the sharp demarcation between the adenomatoid tumor and the adjacent pancreatic acini. The lesion is focally rimmed by lymphocytes (Hematoxylin-eosin, 20×). F, cellular areas are directly adjacent to dense, hyalinized regions (hematoxylin-eosin, 100×). G, the tumor stains diffusely and strongly for CK 5/6 (CK 5/6; 200×). H, positivity for EMA is noted (EMA; 200×).
MATERIALS AND METHODS
Both air-dried and alcohol fixed smears were performed for cytology.
The fresh tissue was formalin fixed, routinely processed, and stained with hematoxylin-eosin.
Immunohistochemistry was performed using the EnVision system (DAKO, Carpenteria, CA). The lesion was stained for the following markers: pancytokeratin (combination of AE1/AE3 [Zymed, San Francisco, CA] and CAM 5.2 [Becton Dickinson, San Jose, CA]), vimentin, S100, neurospecific enolase (Zymed), epithelial membrane antigen (EMA), polyclonal carcinoembryonic antigen, bcl-2, smooth muscle actin, synaptophysin, CD117 (DAKO), CD34 (Becton Dickinson), CK 5/6, calretinin (Biocare Medical, Walnut Creek, CA), chromogranin A and B (ICN Biomedicals, Aurora, OH), and CD 99 (Signet Pathology Systems, Dedham, MA).
Electron microscopy was performed on the paraffin-embedded tissue, but the degree of tissue damage from routine processing obscured meaningful interpretation.
RESULTS
Gross examination revealed a firm, white circumscribed nodule within the pancreatic parenchyma, inferior to the pancreatic duct. The lesion measured 1.7 × 1.6 × 1.5 cm and appeared to have a pushing border. Nine unremarkable lymph nodes were also identified.
Histologically the lesion appeared as a well-circumscribed spindle cell neoplasm with marked stromal hyalinization (Fig. 2D–F). Lymphocytes focally encircled the tumor at its periphery. The neoplastic cells were arranged in solid aggregates, nests, and in small glands all interspersed among the collagen fibers. An attenuated layer of cuboidal cells lined the glands. Cellularity varied from sparse to focally dense. Nuclear shape ranged from spindled to plump with bland and finely granular chromatin with an occasional nucleolus. Rare hyperchromasia and anisonucleosis were noted. Mitotic figures and necrosis were absent.
The tumor cells stained strongly for vimentin, pancytokeratin, EMA, CK 5/6, CD99, and focally for calretinin (Fig. 2G–H). Negative markers included CD34, polyclonal carcinoembryonic antigen, bcl-2, smooth muscle actin, S100, CD117, and neuroendocrine markers.
The preceding fine-needle aspiration yielded an admixture of sparse single cells and loose clusters (Fig. 2A–C). True papillae and glandular configurations were absent. Many bare, naked nuclei were noted. When cytoplasm was identified, it appeared wispy and thin. Nuclei ranged from round to ovoid, with occasional marked anisonucleosis. In some areas, the nuclei were aligned in chain and cords, with rare examples of nuclear molding. Chromatin looked finely granular, and occasional nucleoli were observed.
DISCUSSION
Sections of the pancreatic lesion revealed a firm, white well-circumscribed mass with significant stromal hyalinization. Neoplastic cells arranged in short cords, nests, and blighted ductlike structures percolated among the thick collagen fibers. The nuclei appeared spindled to round with bland, finely granular chromatin. The cells stained for CK 5/6, calretinin, pancytokeratin, EMA, and vimentin. In short, we believe this constellation of cytological and immunohistochemical features is most consistent with an adenomatoid tumor.
These findings compare with previously reported cases, including a solid variant comprised of admixed spindled cells and scattered tubules lined by attenuated cuboidal cells (2, 3, 5). Other common patterns include interlacing cords, tubules, nests, and strands of plump acidophilic cells with cytoplasmic vacuoles (6). Stroma varies from loose connective tissue to dense hyalinized collagen, as seen in our case (6). Like other documented cases, our lesion was partially surrounded by a rim of lymphocytes (2, 7). Seen in 50–80% of cases, associated chronic inflammation is a characteristic feature (2). However, in contrast to some previously reported cases, our lesion displayed discrete encapsulation. Whereas uterine adenomatoid tumors are grossly unencapsulated with indistinct borders (2) and adrenal and pleural lesions appear microscopically infiltrative (3, 4), our pancreatic lesion was surrounded by a distinct, fibrous rim, both grossly and histologically.
Our lesion stained strongly for keratin, vimentin, CK 5/6, and EMA. Staining for calretinin was focal but strong. The lesion was negative for neuroendocrine markers, CD34, bcl-2, smooth muscle actin, CEA, CD117, and S100. This immunohistochemical profile is consistent with the mesothelial origin of an adenomatoid tumor (2, 3, 5, 7, 8). Already well documented in paratesticular adenomatoid tumors (8), the presence of calretinin staining helps support this diagnosis. Likewise, our tumor stained strongly and diffusely for EMA, recapitulating the staining pattern seen in 12/12 paratesticular adenomatoid tumors (5). In sharp contrast, another series noted that 0/60 uterine adenomatoid tumors stained for EMA (2). Data regarding CD99 positivity in adenomatoid tumors are both scant and inconsistent. In one study, 0/3 testicular adenomatoid tumors stained for CD 99 (9), whereas in another, 11/19 mesotheliomas stained for this marker (10). Given this lesion’s mesothelial origin, positive CD99 staining was not surprising. Our lesion also stained for CK 5/6. Because this immunostain is based on selective expression of CK 5 in mesothelial cells, staining 92% of pleural mesotheliomas but only 14% of adenocarcinomas (11), positive staining for CK 5/6 further supports a mesothelial origin. Some report this marker to be as sensitive as calretinin in identifying mesothelial cells (11). However, because we identified no cases in the literature supporting CK 5/6 immunostaining in adenomatoid tumors, further studies are indicated to delineate whether this immunohistochemical profile is specific for adenomatoid tumors or represents aberrant staining in our unusual lesion.
The predominantly solid spindled morphology and hyalinized stroma raised the possibility of other nonepithelial/mesenchymal pancreatic neoplasms. Pancreatic solitary fibrous tumors have been described and can display a similar-appearing fibrous stroma (12). However these lesions characteristically have fascicular and storiform arrangements and lack the tubular structures commonly seen in adenomatoid tumors. Defined by CD34 and bcl-2 immunopositivity, this immunoprofile is inconsistent with mesothelial origin and that of our lesion (13). Solitary fibrous tumors also lack a significant inflammatory component (12). In contrast, the presence of lymphocytes in our lesion prompted consideration of an inflammatory myofibroblastic tumor, also recently described in the pancreas (14). Histologically, the presence of hyalinized fibrosis, numerous lymphocytes, and spindle-shaped fibroblasts recalled this entity. However, our adenomatoid tumor lacked histiocytes, plasma cells, and osteoclast-like giant cells (14). Likewise, inflammatory myofibroblastic tumors stain for smooth muscle actin and vimentin but are negative for CD34, keratin, and EMA (14), again incompatible with our lesion’s immunohistochemical staining profile.
This case is also unique in the addition of retrospective, correlative aspiration cytology. A single case documents the cytologic features of adenomatoid tumors by fine-needle aspiration (15). Fine-needle aspiration of an epididymal adenomatoid tumor yielded sheets, clusters, cords, and a vaguely glandular pattern of monotonous cells with a low N/C ratio (15). These findings correlate well with our depiction of loose chains, cords, and clusters of epithelial cells. Their nuclear details included finely granular, evenly distributed chromatin and small nucleoli. Cytoplasm was described as “pale vacuolated,” which may be likened to our “wispy-appearing” cytoplasm. Although retrospectively consistent with adenomatoid tumor, our results were initially diagnosed as a neuroendocrine neoplasm. Unfortunately, a paucicellular cell block precluded immunohistochemical studies on the aspirate. Clearly, negative neuroendocrine markers would have undermined this cytologic diagnosis.
Correlation between the preceding aspirate and final histology is strong. Both demonstrate the relatively monotonous and bland proliferation of epithelial cells, as seen in side-by-side comparison. Minor discrepancies include the aspirate being more cellular than expected for such a hyalinized lesion and the histology appearing more spindly than the aspirate. Nonetheless, the aspiration results confer a benign diagnosis.
To our knowledge, this is the first report of an adenomatoid tumor in this unusual location. Although this tumor’s immunohistochemical profile supports mesothelial origin and its architecture appears most consistent with that of an adenomatoid tumor, many aspects of this case defy conventional tumor classification. Nonetheless, because this is a benign neoplasm and Whipple procedure certainly would be considered curative, we believe it is important to recognize this tumor in the differential diagnosis of encapsulated pancreatic tumors because prognosis should be excellent.
References
Rosai J . Male reproductive system. In: Rosai J, editor. Ackerman’s surgical pathology.Vol 1. 8th ed. St. Louis, MO: Mosby; 1996: 1300.
Nogales FF, Isaac MA, Hardisson D, Bosincu L, Palacios J, Ordi J, et al. Adenomatoid tumors of the uterus: an analysis of 60 cases. Int J Gynecol Pathol 2002; 21(1): 34–40.
Kaplan MA, Tazelaar HD, Hayashi T, Schroer KR, Travis WD . Adenomatoid tumors of the pleura. Am J Surg Pathol 1996; 20(10): 1219–1223.
Raaf HN, Grant LD, Santoscoy C, Levin HS, Abdul-Karim FW . Adenomatoid tumor of the adrenal gland: a report of four new cases and a review of the literature. Mod Pathol 1996; 9(11): 1046–1051.
Delahunt B, Eble JN, King D, Bethwaite PB, Nacey JN, Thornton A . Immunohistochemical evidence for mesothelial origin of paratesticular adenomatoid tumor. Histopathology 2000; 36: 109–115.
Perez-Ordonez B, Srigley JR . Mesothelial lesions of the paratesticular region. Semin Diagn Pathol 2000; 17(4): 294–306.
Glatz K, Wegmann W . Papillary adenomatoid tumour of the adrenal gland. Histopathology 2000; 37(4): 376–377.
Delahunt B, Eble J, Srigley J, Thornton A . Paratesticular adenomatoid tumor. Assessment of calretinin immunoexpression and cell proliferation indices. J Urol Pathol 2000; 12: 105–115.
Kommoss F, Oliva E, Bittinger F, Kirkpatrick C, Amin M, Bhan A, et al. Inhibin-alpha, CD99, HEA125, PLAP, and chromogranin immunoreactivity in testicular neoplasms and the androgen insensitivity syndrome. Hum Pathol 2000; 31(9): 1055–1061.
Stevenson A, Chatten J, Bertoni F, Miettinen M . CD99 Neuroectodermal/Ewing’s sarcoma antigen as an immunohistochemical marker— review of more than 600 tumors and the literature experience. Appl Immunohistochem 1994; 2(5): 231–240.
Cury PM, Butcher DN, Fisher C, Corrin B, Nicholson AG . Value of the mesothelium-associated antibodies thrombomodulin, cytokeratin 5/6, calretinin, and CD44H in distinguishing epithelioid pleural mesothelioma from adenocarcinoma metastatic to the pleura. Mod Pathol 2000; 13(2): 107–112.
Luttges J, Mentzel T, Hubner G, Kloppel G . Solitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumours. Virchows Arch 1999; 435(1): 37–42.
Luttges J, Reinecke-Luthge A, Mollmann B, Menke MA, Clemens A, Klimpfinger M, et al. Duct changes and K-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch 1999; 435(5): 461–468.
Yamamoto H, Watanabe K, Nagata M, Tasaki K, Honda I, Watanabe S, et al. Inflammatory myofibroblastic tumor (IMT) of the pancreas. J Hepatobiliary Pancreat Surg 2002; 9(1): 116–119.
Rege JD, Amarapurkar AD, Phatak AM . Fine needle aspiration cytology of adenomatoid tumor. A case report. Acta Cytol 1999; 43(3): 495–497.
Acknowledgements
The authors acknowledge Steven Claunch for his technical expertise in immunohistochemistry. We are grateful to Thomas Savides, M.D., for providing the endoscopic ultrasound.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Overstreet, K., Wixom, C., Shabaik, A. et al. Adenomatoid Tumor of the Pancreas: A Case Report with Comparison of Histology and Aspiration Cytology. Mod Pathol 16, 613–617 (2003). https://doi.org/10.1097/01.MP.0000072803.37527.C8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000072803.37527.C8
Keywords
This article is cited by
-
Uterine adenomatoid tumor associated with lymph node lesions: a case report
Abdominal Radiology (2020)
-
Zystischer Tumor an der Leberpforte
Der Pathologe (2013)
-
Leiomyo-adenomatoid tumor of the uterus: a distinct morphological entity?
Archives of Gynecology and Obstetrics (2010)
-
Adenomatoid tumors of the female and male genital tracts: a clinicopathological and immunohistochemical study of 44 cases
Modern Pathology (2009)
-
Mesenchymale Tumoren des Pankreas
Der Pathologe (2005)