Abstract
We reviewed 10 cases of pleomorphic lobular (ductal lobular) carcinoma in situ (PL/DLCIS) of the breast and compared them with 14 cases of pleomorphic lobular carcinoma in situ (PLCIS) found in association with invasive pleomorphic lobular carcinoma. The histologic features; immunohistochemical staining for estrogen receptors (ERs), p53, Ki67, E-cadherin, and gross cystic disease fluid protein-15 (GCDFP-15); and results of fluorescence in situ hybridization for HER-2/neu gene amplification were evaluated in all 24 cases. Histologically, PL/DLCIS cells were similar to those of PLCIS with invasion in that they were discohesive and medium to large in size with moderate to marked nuclear pleomorphism, small to prominent nucleoli, and moderate to abundant eosinophilic or vacuolated cytoplasm. In both groups, central necrosis was present in a small number of cases, and classic LCIS coexisted with the in situ lesion in less than half of the cases; in situ carcinomas were positive for ERs in 23 (100%) of 23 cases, p53 in 6 (25%) of 24 cases, and GCDFP-15 in 14 (74%) of 19 cases. The percentage of Ki67-positive tumor nuclei indicated moderate to high (more than 20%) proliferative activity in 8 (47%) of 17 cases. Immunostaining for E-cadherin was negative in all 24 cases. HER-2/neu gene amplification was observed in 1 (4%) of 23 cases. In cases with associated invasion, PLCIS had cytologic features and immunostaining patterns similar to those of the invasive pleomorphic component. Seven of the 10 patients who had PL/DLCIS without invasion underwent lumpectomy or simple mastectomy. Six of these patients had no evidence of disease in follow-up periods ranging from 4 to 32 months; the seventh patient developed recurrent disease 12 months after undergoing lumpectomy. We conclude that the cytologic features and biomarker expression profile of PL/DLCIS are similar to those of PLCIS with invasion but somewhat different from those of classic LCIS and ductal carcinoma in situ. Long-term follow-up studies are needed to further define the natural history of PL/DLCIS and its optimal management.
Similar content being viewed by others
INTRODUCTION
Carcinoma in situ of the breast is categorized histopathologically as either ductal or lobular depending on its cytologic features and architectural growth pattern. The natural history of each subtype of this disease is different, and patient management varies accordingly. For example, ductal carcinoma in situ (DCIS) is associated with the development of invasive cancer at or near the tumor site and is treated using excision with or without radiotherapy. In contrast, lobular carcinoma in situ (LCIS) appears to be a marker for increased risk of invasive cancer at any site in either breast, and the most common management option for it is lifetime follow-up with or without chemopreventive therapy (1).
Pleomorphic LCIS (PLCIS) is a recently recognized variant of LCIS (2, 3, 4, 5). However, all of the PLCIS cases described in the literature have been associated with infiltrating pleomorphic lobular carcinoma (IPLC) at a frequency of approximately 50% of IPLC cases (2, 3, 4). In those reports, PLCIS was described as being cytologically similar to IPLC with a cell population consisting of large, pleomorphic, dyshesive cells with eccentric nuclei and eosinophilic cytoplasm. Although lesions similar to PLCIS but lacking associated invasion have been encountered, the striking cytologic differences between them and classic LCIS and occasional associated necrosis made distinction of these lesions from solid DCIS difficult. Consequently, such lesions have been designated as indeterminate, mixed ductal lobular carcinoma in situ (DLCIS) (1, 6, 7, 8), or “florid” PLCIS (5). To our knowledge, there have been no reports of PLCIS/DLCIS (PL/DLCIS) as an isolated lesion without associated invasive carcinoma, and information about the natural history of PL/DLCIS is lacking. In addition, the clinical and mammographic presentation and biomarker characteristics of PL/DLCIS are not known.
To address this, we examined a series of 10 cases of PL/DLCIS and 14 cases of PLCIS with associated IPLC and evaluated the clinical presentation, histologic features, and expression of biomarkers involved in cell cycle regulation, cell proliferation, and cellular adhesion. We then compared the features of PL/DLCIS with those of classic LCIS and DCIS.
MATERIALS AND METHODS
We reviewed 10 consecutive cases of PL/DLCIS and 14 cases of PLCIS with associated IPLC retrieved from the surgical pathology files of the Department of Pathology at The University of Texas M. D. Anderson Cancer Center. The cases were diagnosed from 1997 to 2000. Specifically, pathology slides and medical records for all 24 cases were reviewed. Data extracted from the records included mammographic findings, clinical presentation, methods of treatment, and follow-up information. Formalin-fixed, paraffin-embedded tissue blocks obtained from all 24 cases were available for study. For the purpose of better histomorphologic characterization of PL/DLCIS in relation to classic LCIS, 10 consecutive cases of classic LCIS were also retrieved from the surgical pathology file, and their histologic features were reviewed.
The histologic characteristics evaluated included the nuclear size in relation to a lymphocyte, nuclear pleomorphism as graded using a scale of 1–3 (1, mild; 2, intermediate; 3, marked), chromatin pattern (fine versus clumped), presence or absence of nucleoli (including whether they were prominent or small at 10 × magnification), cytoplasmic features (vacuolated versus eosinophilic or granular), loss of cell cohesion, and presence or absence of central necrosis or microcalcifications. The presence or absence of classic LCIS within the same or adjacent lobular units was recorded, as was the presence or absence of associated IPLC and other types of invasive carcinoma. When classic LCIS was present, the cells were categorized as type A, defined as having small, bland, uniform nuclei, or type B, defined as having more abundant cytoplasm and more pleomorphic (grade 1 or 2) nuclei (9).
Replicate tumor sections obtained from the tissue blocks were evaluated via immunohistochemical analysis for estrogen receptors (ERs), p53, Ki67, E-cadherin, and gross cystic disease fluid protein-15 (GCDFP-15) status and via fluorescence in situ hybridization (FISH) for HER-2/neu gene amplification. Briefly, 4-μm-thick tissue sections were cut, mounted on charged slides, deparaffinized in xylene, and rehydrated in ethanol at descending grades (100–70%). For immunohistochemistry, the sections were subjected to heat-induced antigen retrieval via immersion in 0.01 mol/L citrate buffer (pH 6.0), preheated to more than 90°C, and then heated in an electric vegetable steamer (Black and Decker, Shelton, CT) for 54 minutes. Endogenous peroxidase activity was blocked using 5-minute treatment with 3% hydrogen peroxide in absolute methanol.
Immunohistochemical analysis was performed using the avidin-biotin technique (LSAB2 peroxidase kit; Dako Corp., Carpinteria, CA) with primary antibodies and dilutions as summarized in Table 1. The antigen-antibody immunoreaction was visualized using 3,3′-diaminobenzidine as the chromogen, and the slides were counterstained using Mayer’s hematoxylin.
The immunostaining results were evaluated separately for PL/DLCIS with and without invasive carcinoma and for coexisting classic LCIS. Immunoreactivity for ERs, p53, and Ki67 was scored as the estimated percentage of immunostained tumor-cell nuclei. Immunostaining for ERs and p53 was interpreted as being positive when more than 10% of the tumor-cell nuclei showed staining. Also, the proliferation rate was graded as mild, moderate, and marked when less than 10%, 10%-20%, and more than 20% of the tumor-cell nuclei, respectively, were positive for Ki67. Staining for E-cadherin was interpreted as being positive or negative based on the presence or absence of membranous staining of the cells of interest. Finally, staining for GCDFP-15 was interpreted as being positive or negative based on the presence or absence of cytoplasmic staining of the neoplastic cells.
FISH for HER-2/neu gene amplification was performed using the PathVysion HER-2 DNA Probe Kit (Vysis, Downers Grove, IL) according to the manufacturer’s instructions. Briefly, this kit applies two DNA probes directly labeled with different fluorescent dyes: SpectrumOrange fluorophore-labeled LSI HER-2/neu, which is specific for the HER-2/neu gene locus on chromosome 17q12–21.32, and SpectrumGreen fluorophore-labeled CEP 17, which is targeted to the alpha satellite DNA sequence located at the centromeric region of chromosome 17. All of the probes, reagents, and positive controls were purchased from Vysis. The slides were evaluated for the Her-2/neu gene copy number using an epifluorescence microscope (Zeiss, Thornwood, NY).
Signals were counted following the criteria established by Hopman et al. (10). Signals from overlapping nuclei were not counted, and split signals were counted as one chromosome component. The adjacent ductal epithelial cells served as the internal controls. Stromal and inflammatory cells were excluded from analysis based on the morphologic features of their nuclei. Signals from 60 tumor-cell nuclei within the area of interest were counted, and the ratio of total HER-2/neu signals to total CEP 17 signals in all 60 nuclei was calculated. The expected ratio of LSI HER-2/neu to CEP 17 was less than 2 for normal and unamplified tumor tissue specimens; a ratio of 2 or greater was considered to be positive for HER-2/neu gene amplification. The results were reported as amplified or unamplified.
RESULTS
Clinical and Mammographic Findings
All 24 patients were women, and their ages ranged from 36 to 86 years (mean, 55.1 years). Ten of the patients had PL/DLCIS with no associated invasion (age range, 44–64 years; mean age, 50.8 years). Thirteen patients had dominant IPLC in association with PLCIS; while one patient had predominant PLCIS with two microscopic foci (less than 2 mm in extent) of stromal invasion (these 14 patients were categorized as having PLCIS with invasion).
In 9 of the 10 patients who had PL/DLCIS without invasion, the lesions were detected using mammography by the presence of architectural distortions with associated calcifications in four cases, a 5-mm spiculated mass in one case, and calcifications in four cases. The clinical and mammographic findings in the remaining patient were unknown. In the 13 patients having both PLCIS and dominant invasive carcinoma, the clinical presentation was a palpable mass with associated abnormal mammographic findings with or without suspicious calcifications. In the patient having both PLCIS and microinvasion, the lesion was detected using mammography, owing to the presence of calcifications.
Treatment and Follow-up Data
Among the 10 patients having PL/DLCIS without invasion, 5 underwent needle-localization lumpectomy, and 2 underwent simple mastectomy. All seven of these patients are still alive, six of whom had no evidence of recurrent disease (clinically or mammographically) in follow-up periods ranging from 4 to 32 months (mean, 17 months). The seventh patient developed recurrent suspicious calcifications 12 months after initial lumpectomy for PL/DLCIS; in this case, the excised lesion extended to within less than 1 mm from the inked margin of resection. The patient underwent re-excision, which showed in situ carcinoma with features identical to those of the initial lesion. Treatment and follow-up data for the remaining three patients were not available.
The patient having PLCIS and microinvasion underwent simple mastectomy with axillary lymph node dissection. Residual PLCIS with microinvasion was identified in the patient’s breast biopsy site, but her axillary lymph nodes were free of metastasis. She had no evidence of disease at 12 months of follow-up.
The 13 patients having dominant invasive carcinoma and PLCIS underwent segmental resection or mastectomy with or without hormonal therapy, chemotherapy, and/or radiotherapy.
Pathologic Findings
In 7 of the 10 cases of PL/DLCIS without invasion, the macroscopic characteristics of the resected specimens (lumpectomy or simple mastectomy) were reported. The characteristics were described as being unremarkable with no grossly evident lesions.
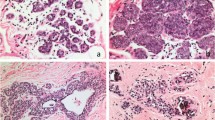
The microscopic features of the 10 PL/DLCIS lesions were generally similar to those of PLCIS with invasion and are summarized in Table 2. In particular, PL/DLCIS involved one or more lobular units and invariably distended the terminal ducts and acini with a pleomorphic population of medium to large cells. The nuclei of PL/DLCIS cells were often ≥4 times the size of a lymphocyte (range, 3–6), and eccentrically placed, and they often exhibited moderate to marked pleomorphism and distinct small or prominent nucleoli (Fig. 1). Additionally, the cytoplasm in these cells was moderate to abundant, usually eosinophilic or granular, and occasionally vacuolated. The cells were evenly spaced and showed focal loss of cohesion in 90% of the cases. Central necrosis with or without calcifications was present in 40% of the cases of PL/DLCIS without invasion and 21% of the cases of PLCIS with invasion; also, microcalcifications were present in 40% of the former and 21% of the latter. PL/DLCIS cells also involved adjacent ducts (pagetoid spread) in the majority of the cases (Fig. 1D).
Examples of PL/DLCIS. Tissue sections obtained from three different PL/DLCIS cases (A and B, case 1; C and D, case 2; E–G, case 3) show lobular distention by a population of medium or large dyshesive cells. The nuclei are pleomorphic and eccentrically placed with occasional prominent nucleoli, and the cytoplasm is moderate to abundant. Of note is the spectrum of cell changes (case 3) with classic LCIS cells (E) at the periphery of the lesion and more large and pleomorphic cells toward the center of the lesion (F). Central necrosis (A) and calcifications (G) are present.
Associated classic LCIS was found in 10 of 24 cases (four [40%] PL/DLCIS cases and six [46%] PLCIS cases with invasion) either admixed with the in situ component or occurring as isolated lobules. Both type A and type B cells were identified. In four cases (two PL/DLCIS, two PLCIS with invasion), classic LCIS was present at the periphery of the areas involved by PL/DLCIS with intervening foci showing transition from small cells having bland nuclear features to more pleomorphic and larger cells (Fig. 1E). In the cases with associated IPLC, the invasive lobular component displayed cytologic features similar to those of the accompanying PLCIS.
In the PL/DLCIS cases without invasion, the histologic findings corresponded to the mass lesion or architectural distortion noted on the mammograms (9 of 10 cases with available mammograms); also, calcifications were noted within the area of PL/DLCIS. PL/DLCIS was not detected in any of the cases as an incidental finding. The excised surgical margins were free of carcinoma and measured at least 3 mm from the tumor in six cases and less than 1 mm in one case.
In the 10 cases of classic LCIS we reviewed, five of the lesions had, in addition to the small, bland, uniform type A cells, cells with plumper nuclei and more abundant cytoplasm: type B cells. A comparison of the cytomorphologic features of PL/DLCIS and classic LCIS is shown in Table 2.
Biomarker Expression Profile and HER-2/neu Status
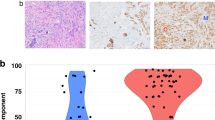
The immunohistochemical staining and FISH results for PL/DLCIS and PLCIS with invasion are summarized in Table 3. All 10 PL/DLCIS cases and 14 PLCIS cases with invasion were positive for ER, with the percentages of positive nuclei ranging from 40–100%. Staining for p53 was positive in 30% of the cases of PL/DLCIS and 29% of the cases of PLCIS with invasion, with the nuclear p53 positivity rate approaching 40% in both p53-positive groups. Additionally, the proliferation rate as demonstrated by Ki67 immunostaining was low in 56% of the cases of PL/DLCIS and 50% of the cases of PLCIS with invasion; the rate was moderate or marked, almost in equal percentages, in the other cases (Fig. 2A). The associated classic LCIS component (n = 10) had a markedly lower percentage of tumor-cell nuclei positive for p53 protein and Ki67 in comparison with the PL/DLCIS or PLCIS components with invasion. Membranous immunostaining for E-cadherin was negative in all of the cases of PL/DLCIS and PLCIS with invasion (Fig. 2B); normal ductal epithelium served as an appropriate internal control. HER-2/neu gene amplification was found in only one case (7%) of PLCIS with invasion and no cases of PL/DLCIS. Finally, immunostaining for GCDFP-15 was performed in 14 cases, 73% of which (PL/DLCIS, 89%; PLCIS with invasion, 60%) were positive.
DISCUSSION
Our findings demonstrate that PL/DLCIS lesions have histomorphologic and biomarker characteristics similar to those of PLCIS with associated invasion. Also, in the cases of PLCIS with invasion, the nuclear and cytoplasmic features of the PLCIS component were remarkably similar to those of the IPLC component. These findings are similar to those described previously (2, 3, 4). Furthermore, our findings support the belief that PL/DLCIS may exist as an isolated lesion and display characteristic cytomorphologic features that allow its recognition.
The histologic features of PL/DLCIS that we observed differ from those of classic LCIS, which is characterized by a population of small, uniform cells and usually lacks necrosis and calcification. In previous studies by Haagensen et al. (9) and Rosen et al. (11), in addition to the characteristic cells of LCIS (type A cells having bland uniform nuclei), some LCIS lesions had type B cells, which were larger and more varied in size and shape. In those series, however, the extent and severity of the changes were not quantitated. Although most of the illustrations in those series showed cells with mild to moderate pleomorphism, it is likely that some of the LCIS lesions having type B cells had a higher level of nuclear pleomorphism similar to that observed in our cases.
In our series, type A and/or B LCIS cells were associated with the PL/DLCIS lesions either individually or within the same space. The features of type B LCIS were generally similar to those of PL/DLCIS except that the cells in the latter had higher nuclear pleomorphism, larger nuclei, and occasional distinct nucleoli. These findings suggest that LCIS represents a spectrum of lesions ranging from those having small uniform cells (grade 1) to those having more nuclear pleomorphism and nuclear enlargement (grades 2 and 3).
When compared with historical biomarker expression data for classic LCIS, PL/DLCIS had a higher proliferation rate and higher percentage of cases showing nuclear accumulation of p53 protein, both features compatible with aggressive behavior (Table 4) (6, 12, 13, 14). Similar to classic LCIS, however, all cases of PL/DLCIS were ER-positive and they all showed uniform loss of membranous staining for E-cadherin. None of the cases showed HER-2/neu gene amplification. The immunostaining positivity for GCDFP-15 seen in most of our PL/DLCIS cases was consistent with the data reported for IPLC (4, 15).
On the other hand, when PL/DLCIS lesions were compared with solid low-and high-grade DCIS, they differed in that DCIS showed E-cadherin expression in the majority of cases and lacked ER expression, especially in high-grade lesions (Table 4) (6, 14, 16). In a study by Shin et al. (17) comparing “florid LCIS with necrosis and calcification” with low-to intermediate-grade solid DCIS, the authors showed that, in contrast to DCIS, all florid LCIS cases displayed mutational alteration of E-cadherin in addition to a lack of E-cadherin expression. In another study by Acs et al. (18), all cases of mixed DLCIS showed a lobular type of E-cadherin expression. Based on their findings, the authors suggested that these lesions are more closely related to LCIS than to DCIS. Similar findings were reported by Jacobs et al. (6) in a subgroup of carcinoma in situ cases that they designated as indeterminate. Our results of the present study are in concordance with those reported previously supporting the closer relationship of PL/DLCIS with LCIS than with DCIS.
The natural history of PL/DLCIS is unknown. This is probably due to the fact that the majority of PL/DLCIS cases, especially those associated with necrosis and calcifications, were categorized as DCIS and therefore treated as such. For example, in National Surgical Adjuvant Breast and Bowel Project protocol B-17, Fisher et al. (7) regarded small-cell lobular lesions with comedo necrosis as “ductal lobular carcinoma in situ” and excluded cases having these lesions from their study of LCIS; in contrast, the same lesions were recently accepted by Tavassoli (19) and Rosen (5) as LCIS. Although the follow-up period in our series was short, most of the patients had no evidence of disease after surgical resection. One patient having PL/DLCIS was found to have recurrent disease 12 months after initial lumpectomy; however, her initial excised lesion was close to the resection margin, and the completeness of the excision was uncertain.
Histologically, PL/DLCIS is differentiated from solid-type DCIS with lobular involvement based on the finding of a population of cells having nuclear and cytoplasmic characteristics of pleomorphic lobular carcinoma: eccentric nuclei, loss of cell cohesion, and negative staining for E-cadherin in neoplastic cells but positive immunostaining in adjacent or residual nonneoplastic epithelium (5, 12, 14, 17, 18). Diffuse positivity for ER regardless of the presence of nuclear pleomorphism and negativity for HER-2/neu gene amplification are additional characteristics that help distinguish PL/DLCIS from high-grade DCIS (Table 4). Furthermore, the presence of associated cells of classic LCIS, including type B cells, and transition from a low to a higher grade of nuclear pleomorphism should support the diagnosis of PL/DLCIS.
Currently, the recommended management of patients having PL/DLCIS is similar to that of patients having DCIS (7, 16). Considering that the majority of PL/DLCIS cases are associated with a mammographic abnormality, we believe that, as with DCIS, complete excision of the lesion with tumor-free margins with or without irradiation is appropriate for patients having PL/DLCIS when possible.
In conclusion, the PL/DLCIS may present as an isolated lesion and displays histomorphologic features and biomarker expression profile similar to those of PLCIS with invasion. Studies using larger numbers of cases with long-term follow-up are needed to further define the biologic behavior of this disease.
References
Schnitt SJ, Morrow M . Lobular carcinoma in situ: current concepts and controversies. Semin Diagn Pathol 1999; 16: 209–223.
Bentz JS, Yassa N, Clayton F . Pleomorphic lobular carcinoma of the breast: clinicopathologic features of 12 cases. Mod Pathol 1998; 11: 814–822.
Frost AR, Tsangaris TN, Silverberg SG . Pleomorphic lobular carcinoma in situ. Pathol Case Rev 1996; 1: 27–31.
Middleton LP, Palacios DM, Bryant BR, Krebs P, Otis CN, Merino MJ . Pleomorphic lobular carcinoma: morphology, immunohistochemistry, and molecular analysis. Am J Surg Pathol 2000; 24: 1650–1656.
Rosen PP . Lobular carcinoma in situ and atypical lobular hyperplasia.In: Rosen’s breast pathology, 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2001. p. 581–626.
Jacobs TW, Pliss N, Kouria G, Schnitt SJ . Carcinoma in situ of the breast with indeterminate features. Am J Surg Pathol 2001; 25: 229–236.
Fisher ER, Costantino J, Fisher B, Palekar AS, Paik SM, Suarez CM, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Cancer 1996; 78: 1403–1416.
Page DL, Anderson TJ . Diagnostic histopathology of the breast. Edinburgh, Scotland: Churchill Livingston, 1987;
Haagensen CD, Lane N, Lattes R, Bodian C . Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer 1978; 42: 737–768.
Hopman AH, Ramaekers FC, Raap AK, Beck JL, Devilee P, van der Ploeg M, et al. In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry 1988; 89: 307–316.
Rosen PP, Lieberman PH, Braun DW Jr . Lobular carcinoma in situ of the breast. Am J Surg Pathol 1978; 3: 225–251.
Krishnamurthy S, Sneige N . Molecular and biological markers of premalignant lesions of human breast. Adv Anat Pathol 2002; 9: 185–197.
Vos CBJ, Cleton-Jansen AM, Berx G, de Leeuw WJ, ter Haar NT, van Roy F, et al. E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer 1997; 76: 1131–1133.
Goldstein NS, Bassi D, Watts JC, Layfield LL, Yaziji H, Gown AM . E-cadherin reactivity of 95 noninvasive ductal and lobular lesions of the breast. Am J Clin Pathol 2001; 115: 534–542.
Eusebi V, Magalhaes F, Azzopardi JG . Pleomorphic lobular carcinoma. Hum Pathol 1992; 23: 665–672.
Maluf HM, Swanson PE, Koerner FC . Solid low-grade in situ carcinoma of the breast: role of associated lesions and E-cadherin in differential diagnosis. Am J Surg Pathol 2001; 25: 237–244.
Shin SJ, DeLellis RA, Knowles DM, Milligan L, Pan L, Rosen PP . “Florid” lobular carcinoma in situ with necrosis and calcification: a clinicopathologic, immunohistochemical and molecular analysis (abstract #206). Mod Pathol 2002; 15: 52A.
Acs G, Lawson TJ, Rebbeck TR, LiVolsi VA, Zhang PJ . Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 2001; 115: 85–98.
Tavassoli FA . Lobular neoplasia (lobular carcinoma in situ). In Pathology of the breast. Norwalk, CT: Appleton & Lange; 1992. p. 263–291.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the United States and Canadian Academy of Pathology, Atlanta, GA, March 2001.
Rights and permissions
About this article
Cite this article
Sneige, N., Wang, J., Baker, B. et al. Clinical, Histopathologic, and Biologic Features of Pleomorphic Lobular (Ductal-Lobular) Carcinoma In Situ of the Breast: A Report of 24 Cases. Mod Pathol 15, 1044–1050 (2002). https://doi.org/10.1097/01.MP.0000027624.08159.19
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000027624.08159.19
Keywords
This article is cited by
-
Immediate and delayed risk of breast cancer associated with classic lobular carcinoma in situ and its variants
Breast Cancer Research and Treatment (2024)
-
Targetable alterations in invasive pleomorphic lobular carcinoma of the breast
Breast Cancer Research (2021)
-
Lobular Neoplasia
Current Breast Cancer Reports (2020)
-
Non-classic LCIS Versus Classic LCIS Versus Atypical Hyperplasia: Should Management be the Same?
Current Surgery Reports (2018)
-
Pleomorphic lobular carcinoma in situ of the breast: a single institution experience with clinical follow-up and centralized pathology review
Breast Cancer Research and Treatment (2017)