Abstract
The distinction between mantle cell lymphoma (MCL) and other small B-cell non-Hodgkin lymphomas (NHL) is important because MCL has a more aggressive clinical course. In bone marrow (BM) biopsy specimens, this distinction can be particularly difficult. Although cyclin D1 immunostaining and molecular detection of the t(11;14) translocation are highly specific markers for MCL, they fail to detect a proportion of cases. We have recently described that MCL typically lacks detectable expression of the cyclin-dependent kinase inhibitor p27kip1 protein by immunostaining, which is expressed at high levels in most small B-cell NHL inversely correlated to the proliferation rate. We therefore examined whether p27kip1 immunostaining could be a useful adjunct for the differential diagnosis of small B-cell NHL infiltrates in the BM. Trephine BM biopsy specimens of 96 patients, including well-characterized MCL (19 cases), B-cell chronic lymphocytic leukemia (27 cases), follicular lymphoma (18 cases), hairy cell leukemia (22 cases), and marginal zone lymphoma (10 cases) as well as 10 reactive BM, including five with benign lymphoid aggregates were investigated. In addition, the presence of a t(11;14) translocation involving the major translocation cluster was studied by PCR in all MCL. All cases of B-cell chronic lymphocytic leukemia, follicular lymphoma, and marginal zone lymphoma revealed a strong p27kip1 nuclear staining in the majority of neoplastic cells. Fourteen (78%) cases of MCL were p27kip1-negative in the tumor cells, whereas four cases revealed a weak nuclear positivity. Seventeen (77%) cases of hairy cell leukemia were also either completely negative for p27kip1 or showed a faint positive staining in a minority of the neoplastic cells. Nine of 19 cases (47%) of MCL showed a bcl1 rearrangement involving the major translocation cluster region. These findings demonstrate that p27kip1 immunostaining is a valuable additional marker for the differential diagnosis of small B-cell NHL infiltrates in BM biopsies. The reduction or lack of p27kip1 protein expression in MCL, as well as in hairy cell leukemia, might be an important event in the pathogenesis of these disorders.
Similar content being viewed by others
INTRODUCTION
Morphologic examination of trephine bone marrow (BM) biopsies is a standard method for staging and follow-up of patients with malignant lymphoma (1, 2, 3, 4, 5, 6). The incidence of BM involvement is highest in small B-cell lymphomas, which also make up the majority of cases where a primary diagnosis of non-Hodgkin lymphoma (NHL) is made on a BM biopsy (4, 5). Small B-cell NHL are a diverse group of clinically, phenotypically and genotypically well defined disease entities (7). Despite this fact, a definite separation of these lymphoma subtypes on BM trephine biopsies remains difficult, due to overlapping cytologic features and patterns of distribution of the neoplastic infiltrates (2). From this group of lymphoproliferations with a predominance of small cells, mantle cell lymphoma (MCL) stands out as a more aggressive neoplasm with poor response to conventional therapeutic regimens and a median survival duration of 3 to 4 years (8, 9). Like other small B-cell NHL, the majority of patients present with advanced disease, including generalized lymphadenopathy and BM involvement (8, 10, 11, 12). Therefore, the distinction of MCL from other B-cell neoplasms in BM biopsies is of significant clinical relevance. Immunohistochemical demonstration of cyclin D1 protein is an important marker for the diagnosis of MCL, because it is expressed neither in normal lymphocytes nor in most B-cell NHL (6, 8, 13, 14). However, cyclin D1 staining can be capricious in decalcified BM biopsies, and false negative results may lead to misclassification of lymphoma infiltrates (6, 15, 16).
p27kip1 is a cyclin-dependent kinase inhibitor which is crucial for cell cycle progression from G1 into S phase (17, 18). Deregulation of p27Kip1 expression is a relatively common feature in many solid tumors, but alterations of the gene are infrequent (19, 20, 21, 22). We and others have recently observed that the lack or reduction of p27Kip1 protein expression is a characteristic feature of MCL, in contrast to other small B-cell NHL, in which p27Kip1 is strongly expressed (23, 24, 25). We therefore investigated the utility of p27Kip1 immunostaining for the differential diagnosis of small B-cell NHL infiltrates in routinely processed BM biopsies.
MATERIALS AND METHODS
Tissue Samples
Trephine BM biopsy specimens of 96 patients were selected from the files of the Institutes of Pathology of the University of Innsbruck, Austria, the Technical University of Munich, Germany, and the General Hospital Salzburg, Austria. All cases were classified according to the Revised European-American Lymphoma and the upcoming WHO classifications (7, 26). They included 19 cases of MCL, of which 18 were classified as typical MCL and 1 as blastic variant of MCL (27), 27 cases of B-cell chronic lymphocytic leukemia (B-CLL)/small lymphocytic leukemia (SLL), 18 cases of follicular lymphoma (FL) (grades I and II), 22 cases of hairy cell leukemia (HCL), and 10 cases of marginal zone lymphoma (MZL). As controls, 10 BM biopsies with reactive changes, including five BM biopsies with reactive nodular lymphoid infiltrates, partially with germinal centers were analyzed. Additional lymph node (LN) biopsies were investigated in five cases of MCL.
The primary diagnosis of MCL, FL, and MZL had been made on LN biopsies or extranodal tumor infiltrates thoroughly characterized by paraffin section immunostaining. For a diagnosis of HCL, expression of CD103 was demonstrated on BM frozen sections, in addition to standard morphologic criteria and paraffin immunostaining including monoclonal antibody DBA 44 (28, 29, 30). Cases of B-CLL/SLL had been immunophenotyped by flow cytometry of peripheral blood or BM samples.
BM samples were formaldehyde (4%) fixed (pH 7.4) for at least 24 hours and decalcified with buffered sodium-ethylenediaminetetra-acetic acid (pH 7.0) for 48 hours. Four to five μm thick sections were cut, stained with hematoxylin and eosin, Giemsa, periodic acid-Schiff, Gomori's reticulin stain, and Naphtol AS-D chloroacetate esterase.
Immunohistochemistry
All cases were stained for cyclin D1 (clone P2D11F11, Novocastra, Newcastle, UK; dilution 1:10), CD20 (DAKO, Copenhagen, Denmark; dilution 1:500) and polyclonal CD3 (DAKO; dilution 1:200). The expression of p27Kip1 was investigated with the monoclonal antibody Kip-1 (Transduction Laboratories, Lexington, KY; dilution 1:1000). Moreover, in selected cases stains for CD10 (Novocastra; dilution 1:10), CD5 (clone 4C7, Novocastra; dilution 1:50), CD23 (Novocastra; dilution 1:50), and MiB-1 (Dianova, Hamburg, Germany) were accomplished.
Immunohistochemistry was performed on an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's protocols, with minor modifications (24). After deparaffinization and rehydration, the slides were placed in a microwave pressure cooker in 0.01 mol/L citrate buffer (pH 6.0) containing 0.1% Tween 20 and heated in a microwave oven at maximum power (800W) for 35 minutes. The sections were immediately cooled in Tris-buffered saline and washed in 3% goat serum for 20 minutes. Incubations with the primary antibodies were performed overnight at room temperature. The rest of the procedure was completed on the Ventana immunostainer.
Positive controls for all investigated antibodies were used to confirm the adequacy of the staining. The staining quality of cyclin D1 was verified by a cyclin D1-positive MCL, carrying a t(11;14) translocation. Furthermore, scattered positive endothelial cells served as internal control. For the comparison of p27Kip1 staining between tissue samples, T-cells were used as internal controls.
Double immunostaining was also performed on representative cases to more precisely evaluate the coexpression of p27Kip1 with B- (CD20) and T- (CD3) cell markers. For these reactions, p27Kip1 was detected as described above followed by 2 hours of incubation with CD20 or CD3 antibodies, respectively. The second reaction was developed using the Elite-ABC-Kit and VIP substrate (Vector laboratories, Burlingame, CA, USA) resulting in a contrasting dark purple precipitate.
All immunohistochemical stainings were reviewed by three pathologists (MK, LQ-M, FF). Because cyclin D1 expression is undetectable in normal lymphatic cells, all unequivocal, nuclear staining in tumor cells was classified as positive. For p27Kip1 protein expression, the staining intensity was graded as negative, weak, moderate and strong; strong staining was defined as being of comparable intensity to reactive T-cells and plasma cells. A lymphoma was considered as p27Kip1-positive, if at least 20% of the neoplastic cells showed any nuclear staining.
Detection of bcl1 Translocations by PCR Analysis
In all MCL cases and in the HCL cases showing positivity for cyclin D1, the presence of translocations involving the bcl1/JH major translocation cluster (MTC) was assessed by PCR using a previously published protocol (31). Briefly, DNA was extracted from paraffin-embedded BM and LN biopsy specimens. The tissue was dewaxed, and digested by overnight proteinase K incubation (20 mg/mL) at 55°C. Amplification was performed using a consensus JH primer 5′-ACC TGA GGA GAC GGT GAC CAG GGT-3′ and one of two MTC region primers, MTC1 5′-CCT CTC TCC AAA TTC CTG-3′ and MTC2 5′-GAT GGG CTT CTC TCA CCT ACT A-3′. Forty cycles of amplification under previously established, optimized conditions were performed. The product was electrophoresed in a 2% agarose gel, stained with ethidium bromide, and photographed under ultraviolet light. Selected positive cases were analyzed by direct sequencing to confirm the specificity of the amplification product. Stringent laboratory protocols were followed to prevent PCR product contamination. A well characterized MCL served as positive control, and was included in every run.
RESULTS
Immunohistochemical Findings
P27Kip1 Protein Expression in Reactive BM Samples
In normal BM, p27Kip1 protein was expressed in the nuclei of most megakaryocytes, as well as in endothelial cells, plasma cells and small reactive T-lymphocytes. All other hematopoietic cells were negative for p27Kip1 (32). Reactive nodular lymphoid infiltrates showed strong p27Kip1 protein expression, whereas germinal centers where negative for p27Kip1.
p27Kip1 and Cyclin D1 Protein Expression in MCL
Fourteen of the 18 cases (78%) of classical MCL were classified as negative for p27Kip1, showing either a faint reactivity of tumor cells in two cases (15%) or complete lack of staining in 12 cases (85%). In all cases, scattered small lymphocytes strongly positive for p27Kip1 were identified. Serial sections stained for CD3, as well as double stainings revealed that these p27Kip1-positive small lymphocytes were reactive T cells (Fig. 1, A–D). In the remaining four cases, the majority of the neoplastic cells were weakly p27Kip1-positive, at a significantly lower intensity than the scattered T cells. The single case of blastic variant of MCL weakly expressed p27Kip1 in most tumor cells despite a high proliferation index as assessed by MiB1 staining (more than 80%). Nuclear cyclin D1 expression was demonstrated in all but two cases (89%). However, the staining was weak and heterogeneous in many BM biopsies, making interpretation problematical. Both MCL cases negative for cyclin D1 in the BM were cyclin D1 positive in the diagnostic LN biopsy examined in parallel. In addition, one of these two cases showed a bcl1 rearrangement by PCR (Table 1). Both cases were p27Kip1-negative in the BM, supporting a diagnosis of MCL.
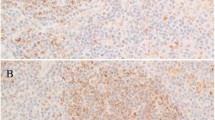
p27Kip1 expression in small B-cell NHL infiltrates in the BM. A–D, BM with prominent infiltrate of a typical MCL. A, H&E; × 200. B, The neoplastic cells are predominantly CD20-positive B-cells. (Immunoperoxidase); × 300. C, CD3 staining reveals a considerable number of infiltrating CD3-positive T-cells. (Immunoperoxidase); × 300. D, p27Kip1 staining. The neoplastic cells show the characteristic lack of p27Kip1 expression, whereas the intermingled T-lymphocytes are strongly positive. (Immunoperoxidase); × 200. Insert: double staining for p27Kip1 (brown) and CD3 (purple) in the same infiltrate of MCL demonstrates that the p27Kip1-positive cells within the tumor co-express CD3. (Immunoperoxidase); × 600. E–G, CD20 and p27Kip1 expression in a case of B-CLL in the BM. E, H&E: × 200. F, The majority of neoplastic B-cells express membranous CD20. (Immunoperoxidase); × 300. G, Strong nuclear p27Kip1 expression in nearly all tumor cells, similar to the intensity seen in T-lymphocytes. (Immunoperoxidase); × 200. H–J, BM with a typical infiltrate of HCL. H, H&E: × 200. I, The hairy cells show a characteristic strong membranous CD20 positivity. (Immunoperoxidase); × 300. J, The neoplastic hairy cells are completely negative for p27Kip1, whereas some reactive T-lymphocytes and plasma cells are positive. (Immunoperoxidase); × 200.
p27Kip1 and Cyclin D1 Protein Expression in NHLs Other than MCL
A total of 77 cases of B-cell NHLs other than MCL were also immunostained for p27Kip1 and cyclin D1. The results are summarized in Table 2. All cases of B-CLL (27 cases), FL (18 cases), and MZL (10 cases) revealed a strong p27Kip1 nuclear staining in the vast majority of tumor cells, similar to the intensity seen in T lymphocytes (Fig. 1, E–G). Occasional transformed cells in FLs and B-CLLs were p27Kip1-negative. Cyclin D1 protein was undetectable in all examined cases of these lymphoma subtypes.
HCL represented a distinct group set apart from the other small B-cell NHL. Five of 22 cases (23%) showed a weak to moderate p27Kip1 reactivity in more than 50% of the neoplastic cells, but less strong than plasma cells and scattered T lymphocytes (Figure 1, H–J). Seventeen cases (77%) of HCL were classified as negative for p27Kip1, showing either a faint reactivity in rare cells (eight cases), or complete lack of staining in neoplastic cells (nine cases) (Table 2). The proliferation activity of neoplastic cells was very low, as assessed by immunostaining for MiB1.
Cyclin D1 protein expression was detectable in nine cases (41%). Three cases (13%) showed a weak to moderate nuclear staining in the majority of the tumor cells, whereas six cases (27%) revealed a mostly weak nuclear staining in a minority of the neoplastic cells.
Molecular Analysis
PCR amplification of DNA extracted from tissues of the 19 MCL cases revealed strong bands of appropriate size in 9 (47%) of 19 cases, confirming the presence of a bcl1 translocation involving the MTC (Fig. 2). In Cases 2 and 3 (Table 1), PCR failed to detect the bcl1 rearrangement in the BM and LN, respectively, irrespective of a reproducible positive signal in the additionally investigated tissues from the same patients. This discrepancy is most probably due to insufficient DNA quality in the formalin-fixed samples, because the expected product sizes are between 370 and 600 base pairs. As expected, the investigated nine HCL failed to show a bcl1 translocation.
Detection of bcl1 translocations involving the MTC region in BM biopsies by PCR analysis. A, PCR amplification with the MTC-1 primer. MW: fragment size marker; C: MCL with a bcl1 translocation confirmed by sequencing as positive control; N: negative control. Lanes 1–4 show bands of appropriate size for Cases 1, 3, 5, and 6, whereas Lanes 5–7 show negative results for Cases 10–12. B, PCR amplification with the MTC-2 primer of the same cases as shown in A; Lanes 1–4 (Cases 1, 3, 5, and 6) show strong bands of the expected size. Cases 10–12 were also negative, as shown for the MTC-1 region.
DISCUSSION
Our study demonstrates that immunohistochemical detection of p27Kip1 protein is a valuable marker for the differential diagnosis of B-cell NHL infiltrates in BM biopsies. The consistent lack of p27Kip1 protein expression allows the distinction of MCL from other small B-cell NHL, which constantly show a strong p27Kip1 reactivity with the notable exception of HCL. However, p27Kip1 protein expression does not discriminate between reactive and neoplastic lymphoid nodular infiltrates in the BM, with the exception of MCL.
The classification of small B-cell lymphomas in the BM is a common diagnostic problem resulting from overlapping morphologic features (1, 3, 6, 15). The distinction of MCL from other entities is clinically important because of its poor response to therapy and its unfavorable prognosis (8). Since of the description of cyclin D1 overexpression as a consistent feature of MCL and the availability of antibodies suitable for paraffin-embedded tissue, immunostaining for cyclin D1 has become an important adjunct for the diagnosis of MCL (8, 13, 15, 33). Nevertheless, immunohistochemical demonstration of cyclin D1 can be capricious, especially in routinely processed and decalcified BM biopsy specimens, and a negative result does not rule out a diagnosis of MCL (6, 16). Even in well fixed and paraffin-embedded LN biopsies, immunohistochemistry fails to detect cyclin D1 protein in 10 to 30% of MCL (6, 15). In addition, the choice of fixative and decalcification procedure for BM biopsies is critical for cyclin D1 staining. Fixation in a 1% formaldehyde solution containing 0.4% glutaraldehyde, a BM fixative widely used in Germany, completely abolishes staining for cyclin D1 (data not shown). Although molecular demonstration of a bcl1 rearrangement can resolve some of the cases, PCR and even Southern blot analysis fail to identify a significant number of cases due to the wide distribution of breakpoints outside the MTC (34). Amplification of the MTC by PCR renders a positive result only in 33 to 50% of MCL cases in paraffin-embedded specimens (31, 35, 36, 37). Our detection rate of 47% is consistent with these published data.
Keeping these diagnostic difficulties in mind, we decided to analyze whether the characteristic lack of p27Kip1 immunostaining in MCL can be exploited for the differential diagnosis of BM biopsies involved in lymphoma (24, 25).
In accordance with our previous study, a lack or significant reduction of p27Kip1 immunostaining in a CD5-positive small B-cell neoplasia is highly suggestive of MCL, whereas a strong, homogeneous expression practically rules out this diagnosis (24). The usefulness of additional p27Kip1 staining for a diagnosis of MCL is documented by two of our cases, which were negative for cyclin D1 in the BM. In both of them, the diagnosis of MCL was confirmed by a positive cyclin D1 staining in the additionally investigated primary LN biopsy. Both of them showed a negative p27Kip1 staining in the BM, underlining the value of this antibody for difficult cases. However, the strong positivity of reactive T-cells has to be taken into consideration for a correct interpretation of p27Kip1 stains. For small B-cell NHL infiltrates with a high number of infiltrating reactive T-lymphocytes, careful comparison with an adjacent section stained for T-cells is necessary.
Whereas MCL characteristically shows absence or significant reduction of immunodetectable p27Kip1, with the exception of the blastic variant of MCL, small B-cell NHL show high expression of p27Kip1 in strictly inverse correlation to the proliferation rate (23, 24, 25). The absence of p27Kip1 protein in MCL is not due to gross rearrangements or deletion of the p27Kip1 gene, and the mRNA levels of p27Kip1 are in a normal range (24, 25). Recently, it has been proposed that an increased degradation of p27Kip1 protein via the proteasome pathway might be an explanation for this phenomenon (25).
Unexpectedly and similar to MCL, HCL lacks p27Kip1 expression irrespective of a generally very low proliferation rate. In our study, the majority of HCL cases (77%) showed low or undetectable levels of p27Kip1, and in the remaining positive cases the intensity of immunostaining was less than in the reactive T- and plasma cells. This finding confirms a recently published study by Chilosi et al., who investigated 58 HCL and found a lack of p27Kip1 protein expression in nearly all of them (93%) (38). The reason for the lack of p27Kip1 protein expression in HCL is unclear. In contrast to MCL and epithelial neoplasms, no increased p27Kip1 degradation was observed (25, 38). A common feature of both MCL and HCL is cyclin D1 protein overexpression. There is a considerable variation in the rate of cyclin D1 positivity reported for HCL, ranging from as little as 6% to 100% in different studies (6, 14, 39, 40). These results are probably due to the use of different antibodies and antigen retrieval techniques. Our rate of 41% positivity for cyclin D1 is in accordance with these data. In contrast to MCL, overexpression of cyclin D1 in HCL is not due to alterations involving the 11q13 region (39, 41, 42). Whether the overexpression of cyclin D1 in these two otherwise distinct B-cell neoplasms could hint to common mechanisms responsible for the absence or reduction of immunodetectable p27Kip1 protein remains to be determined. In terms of differentiation between MCL and HCL, the common lack of p27kip1 expression should not pose diagnostic problems, due to the characteristic morphology and CD5 negativity of HCL.
CONCLUSION
In conclusion, our findings demonstrate that the consistent lack of p27kip1 protein expression allows the distinction of MCL from other small B-cell NHL, making p27kip1 immunostaining a valuable additional marker for the differential diagnosis of small B-cell NHL infiltrates in BM biopsies.
References
Thiele J, Langohr J, Skorupka M, Fischer R . Reticulin fiber content of bone marrow infiltrates of malignant non-Hodgkin's lymphomas (B-cell type, low malignancy): A morphometric evaluation before and after therapy. Virchows Arch A Pathol Anat Histopathol 1990; 417: 485–492.
Schmid C, Isaacson PG . Bone marrow trephine biopsy in lymphoproliferative disease. J Clin Pathol 1992; 45: 745–750.
Coad JE, Olson DJ, Christensen DR, Lander TA, Chibbar R, McClennen RC, et al. Correlation of PCR-detected clonal gene rearrangements with bone marrow morphology in patients with B-lineage lymphomas. Am J Surg Pathol 1997; 21: 1047–1056.
Crotty PL, Smith BR, Tallini G . Morphologic, immunophenotypic, and molecular evaluation of bone marrow involvement in non-Hodgkin's lymphoma. Diagn Mol Pathol 1998; 7: 90–95.
Brunning RD, McKenna RW . Tumors of the bone marrow. In: Rosai J, Sobin LH, editors. Atlas of Tumor Pathology. 3rd series, vol 9. Washington, DC: Armed Forces Institute of Pathology, 1994: 255–292.
Vasef MA, Medeiros LJ, Koo C, McCourty A, Brynes RK . Cyclin D1 immunohistochemical staining is useful in distinguishing mantle cell lymphoma from other low-grade B-cell neoplasms in bone marrow. Am J Clin Pathol 1997; 108: 302–307.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood 1994; 84: 1361–1392.
Campo E, Raffeld M, Jaffe ES . Mantle-cell lymphoma. Semin Hematol 1999; 36: 115–127.
Banks PM, Chan J, Cleary ML, Delsol G, De Wolff-Peeters C, Gatter K, et al. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic and molecular data. Am J Surg Pathol 1992; 16: 637–640.
Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD . Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997; 89: 2067–2078.
Fisher RI, Dahlberg S, Nathwani BN, Banks PM, Miller TP, Grogan TM . A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group Study. Blood 1995; 85: 1075–1082.
Norton AJ, Matthews J, Pappa V, Shamash J, Rohatiner AZS, Lister TA . Mantle cell lymphoma: natural history defined in a serially biopsied population over a 20-year period. Ann Oncol 1995; 6: 249–253.
Rosenberg CL, Wong E, Petty EM, Bale AE, Tsujimoto Y, Harris NL, et al. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci USA 1991; 88: 9638–9642.
Zuckerberg LR, Yang WI, Arnold A, Harris NL . Cyclin D1 expression in non-Hodgkin's lymphomas. Am J Clin Pathol 1995; 103: 756–760.
Chan JKC . Immunostaining for cyclin D1 and the diagnosis of mantle cell lymphoma: is there a reliable method? Histopathology 1999; 34: 266–268.
Miller KD, Munson P, Isaacson PG . Optimizing cyclin D1 immunostaining of mantle cell lymphoma. Histopathology 1999; 34: 268–270.
Hirama T, Koeffler HP . Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood 1995; 86: 841–854.
Sherr CJ, Roberts JM . CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13: 1501–1512.
Esposito V, De Baldi A, Luca A, Groger AM, Loda M, Giordano GG, et al. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997; 57: 3381–3385.
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, et al. Increased proteasome-dependent degradation of the cyclin dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nature Med 1997; 3: 231–234.
Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galuzzi C, et al. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a, b) invasive breast carcinomas. Cancer Res 1997; 57: 1259–1263.
Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, de Kernion JB, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. Urology 1998; 159: 941–945.
Sanchez-Beato M, Saez AI, Martinez-Montero JC, Mateo MS, Sanchez-Verde L, Villuendas R, et al. Cyclin-dependent kinase inhibitor p27kip1 in lymphoid tissue: p27 kip1 expression is inversely proportional to the proliferative index. Am J Pathol 1997; 151: 151–160.
Quintanilla-Martínez L, Thieblemont C, Fend F, Kumar S, Pinyol M, Campo E, et al. Mantle cell lymphomas lack expression of p27/kip1, a cyclin-dependent kinase inhibitor. Am J Pathol 1998; 153: 175–182.
Chiarle R, Budel LM, Skolnik J, Frizzera G, Chilosi M, Corato A, et al. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood 2000; 95: 619–626.
Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK . World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. A progress report. Am J Clin Pathol 1999; 111 (1 Suppl): S8–S12.
Ott G, Kalla J, Ott MM, Schryen B, Katzenberger T, Müller JG, et al. Blastoid variants of mantle cell lymphoma: frequent bcl-1 rearrangements at the major translocation cluster region and tetraploid chromosome clones. Blood 1997; 89: 1421–1429.
Thaler J, Dietze O, Faber V, Greil R, Gastl G, Denz H, et al. Monoclonal antibody B-ly7: a sensitive marker for detection of minimal residual disease in hairy cell leukemia. Leukemia 1989; 4: 170–176.
Salomon-Nguyen F, Valensi F, Troussard X, Flandrin G . The value of the monoclonal antibody, DBA.44, in the diagnosis of B-lymphoid disorders. Leukemia Res 1996; 20: 909–913.
Hounieu H, Chittal SM, Al Saati T, De Mascarel A, Sabattini E, Pileri S, et al. Hairy cell leukemia. Diagnosis of bone marrow involvement in paraffin-embedded sections with monoclonal antibody DBA.44. Am J Clin Pathol 1992; 98: 26–33.
Fan H, Gulley ML, Gascoyne RD, Horsman DE, Adomat SA, Cho CG . Molecular methods for detecting t(11;14) translocations in mantle-cell lymphomas. Diagn Mol Pathol 1998; 7: 209–214.
Taniguchi T, Endo H, Chikatsu N, Uchimaru K, Asano S, Fujita T, et al. Expression of p21/cip1/waf1/sdi1 and p27/kip1 cyclin-dependent kinase inhibitors during human hematopoiesis. Blood 1999; 93: 4167–4178.
Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, et al. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood 1994; 84: 2726–2732.
Williams ME, Meeker TC, Swerdlow SH . Rearrangement of the chromosome 11 bcl-1 locus in centrocytic lymphoma. Analysis with multiple breakpoint probes. Blood 1991; 78: 493–498.
Segal GH, Maiese RL . Mantle cell lymphoma: rapid polymerase chain reaction-based genotyping of a morphologically heterogeneous entity. Arch Pathol Lab Med 1996; 120: 835–841.
Lim LC, Segal GH, Wittwer CT . Detection of bcl-1 gene rearrangement and B-cell clonality in mantle cell lymphoma using formalin-fixed, paraffin embedded tissues. Am J Clin Pathol 1995; 104: 689–695.
Lasota J, Franssila K, Koo CH, Miettinen M . Molecular diagnosis of mantle cell lymphoma in paraffin-embedded tissue. Mod Pathol 1996; 9: 361–366.
Chilosi M, Chiarle R, Lestani M, Menestrina F, Montagna L, Ambrosetti A, et al. Low expression of p27 and low proliferation index do not correlate in hairy cell leukaemia. Br J Haematol 2000; 111: 263–271.
de Boer CJ, Kluin-Nelemans JC, Dreef E, Kester MG, Kluin PM, Schuuring E, et al. Involvement of the CCND1 gene in hairy cell leukemia. Ann Oncol 1996; 7: 251–256.
Miranda RN, Briggs RC, Kinney MC, Veno PA, Hammer RD, Cousar JB . Immunohistochemical detection of cyclin D1 using optimized conditions is highly specific for mantle cell lymphoma and hairy cell leukemia. Mod Pathol 2000; 13: 1308–1314.
Bosch F, Campo E, Jares P, Pittaluga S, Munoz J, Nayach I, et al. Increased expression of the PRAD-1/CCND1 gene in hairy cell leukemia. Br J Haematol 1995; 91: 1025–1030.
Sola B, Salaün V, Ballet JJ, Troussard X . Transcriptional and post-transcriptional mechanisms induce cyclin D1 over-expression in chronic lymphoproliferative disorders. Int J Cancer 1999; 83: 230–234.
Acknowledgements
This work was in part supported by a grant from the Wilhelm Sander-Stiftung, Germany.
The authors thank Birgit Geist, Jaqueline Mueller, and Elonore Samson for their expert technical assistance, and Dr. Otto Dietze (General Hospital, Salzburg, Austria) for providing us with excellent archive material.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Quintanalla-Martínez and Fend contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kremer, M., Dirnhofer, S., Nickl, A. et al. p27Kip1 Immunostaining for the Differential Diagnosis of Small B-Cell Neoplasms in Trephine Bone Marrow Biopsies. Mod Pathol 14, 1022–1029 (2001). https://doi.org/10.1038/modpathol.3880429
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880429