Abstract
Chromophobe renal carcinoma is composed of neoplastic cell showing several features similar to those found in the intercalated cells of the collecting ducts. Because the distal nephron expresses calcium-binding proteins playing a role in calcium homeostasis, we reasoned that these proteins could be expressed by chromophobe carcinoma and therefore represent a diagnostic marker. We studied the immunohistochemical expression of different calcium-binding proteins (parvalbumin, calbindin-D28K, and calretinin) in 140 renal tumors, including 75 conventional (clear cell) carcinomas, 32 chromophobe carcinomas, 17 papillary renal cell carcinomas, and 16 oncocytomas. Parvalbumin was strongly positive in all primary chromophobe carcinomas and in one pancreatic metastasis; it was positive in 11 of 16 oncocytomas and absent in conventional (clear cell) and papillary renal cell carcinomas, either primary or metastatic. Calbindin-D28K and calretinin were negative in all tumors, with the exception of two chromophobe carcinomas, four oncocytomas, and two papillary renal cell carcinomas showing inconspicuous calretinin expression. Our data demonstrate that parvalbumin may be a suitable marker for distinguishing primary and metastatic chromophobe carcinoma from conventional (clear cell) and papillary renal cell carcinoma. Moreover, they suggest a relationship between chromophobe renal carcinoma and renal oncocytoma and indicate that chromophobe carcinoma exhibits differentiation toward the collecting-duct phenotype.
Similar content being viewed by others
INTRODUCTION
The differential diagnosis of renal tumors is of high clinical relevance. The recent classifications have greatly helped in this issue by clearly defining the major subtypes of kidney epithelial tumors, according to reproducible morphologic and cytogenetic criteria (1, 2).
Oncocytomas are benign tumor, whereas chromophobe carcinoma appears to behave more indolently than papillary renal cell and conventional (clear cell) carcinomas (3). However, a certain degree of morphologic and immunophenotypic overlap exists among these neoplasms, the chromophobe carcinoma being the more problematic to diagnose.
Chromophobe carcinoma accounts for 5% of epithelial tumors of the kidney and is considered to have low malignant potential (4, 5, 6). It has been suggested that the cells of chromophobe renal carcinoma are related to the normal intercalated cells of the collecting ducts, as it is ultrastructurally characterized by the presence of numerous cytoplasmic vesicles resembling those observed in these cells (5, 6). Two subtypes of chromophobe carcinoma have been described, a typical variant and an eosinophilic variant (5). The first has to be distinguished from conventional (clear cell) carcinoma, whereas the second must be separated from those tumors characterized by cells with granular cytoplasm, such as renal oncocytomas, conventional (clear cell) carcinomas with diffuse areas of granular cells, as well as some rare forms of papillary renal cell carcinoma.
Several features have been proposed to accurately recognize chromophobe carcinoma, including histochemical, immunohistochemical, genetic, and ultrastructural findings (4, 8, 9, 10, 11, 12, 13, 14). In particular, chromophobe renal carcinoma is characteristically stained by Hale's colloidal iron (10, 12), immunoreacts with anti-E-cadherin (14) and antimitochondrial 113-1 (11) antibodies, and shows hypodiploidy caused by frequent loss of many different chromosomes (8, 9).
Nevertheless, all of these features are limited for practical histopathology, and the availability of other cell markers is warranted to better distinguish different renal epithelial tumors.
As the distal nephron expresses calcium-binding proteins playing a role in calcium homeostasis (15), we reasoned that these proteins could be expressed by chromophobe renal carcinoma and therefore represent a diagnostic marker.
In this study, we investigated the immunohistochemical expression of three calcium-binding proteins, parvalbumin, calbindin-D28K, and calretinin (16, 17, 18, 19, 20, 21), in a large series of renal epithelial tumors, including all four major subtypes. Our data show that parvalbumin is a highly specific and reliable marker for distinguishing chromophobe renal carcinoma from other renal carcinoma types. In addition, we furnish support to the evidence that chromophobe renal carcinoma shows differentiation toward collecting duct phenotype by the demonstration that parvalbumin is expressed in the collecting ducts of fetal and adult kidney.
MATERIALS AND METHODS
We studied the immunohistochemical expression of parvalbumin, calbindin-D28K, and calretinin in 140 renal epithelial tumors. One hundred were consecutive neoplasms, including 75 conventional (clear cell) renal carcinomas, 6 chromophobe renal carcinomas, 17 papillary renal cell carcinomas, and 2 oncocytomas. These tumors were collected from the file of the Department of Pathology of the University of Verona between 1990 and 1992. The other 40 cases, 26 chromophobe carcinomas and 14 oncocytomas, were included on the basis of the preliminary results. All tumors were classified according to the criteria of the current classifications (1, 2), and all carcinomas were graded according to the morphologic parameters proposed by Fuhrman (22). The expression of these three proteins was also evaluated in five fetal kidneys and in unaffected samples of the kidneys harboring the tumors.
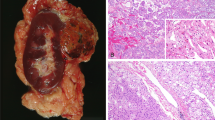
Conventional (clear cell) carcinomas showed nuclear Grade 1 in 10 cases, Grade 2 in 30 cases, Grade 3 in 30 cases, and Grade 4 in five cases. Granular areas were present in 20 cases. There were thirteen Type 1 and four Type 2 papillary renal cell carcinomas. Nuclei were Grade 1 in five cases, Grade 2 in five cases, Grade 3 in six cases, and Grade 4 in one case. Chromophobe renal carcinomas had their cells arranged predominantly in a solid pattern in 22 cases, a tubuloalveolar pattern in seven cases, and a nested pattern in three cases. All chromophobe carcinomas contained a variable admixture of the three cell types identified by Akhtar et al. (23). Twenty chromophobe carcinomas were classified as classic type (Fig. 2A), as they were predominantly composed of large polygonal cells with abundant, pale, reticulated cytoplasm and with well defined cytoplasm borders (Type 3 cells). Twelve were considered eosinophilic variants of chromophobe renal carcinomas (Fig. 2C), as their predominant cell type was characterized by abundant eosinophilic cytoplasm denser at the periphery and a perinuclear clearing (Type 2 cells) in 10 cases and by small, solid, and slightly granular cytoplasm without significant translucent areas (Type 1 cells) in two cases. Microcalcifications were found in 15 tumors.
A, chromophobe carcinoma, classic variant prevalently composed by large polygonal cells with abundant reticulated cytoplasm (Type 3 cells according to Akhtar et al.; 1). B, strong and diffuse parvalbumin immunohistochemical expression in chromophobe carcinoma, classic variant. C, chromophobe carcinoma, eosinophilic variant, prevalently composed of cells with granular and eosinophilic cytoplasm (Type 1 cell according to Akhtar et al.; 1). D, strong and diffuse parvalbumin immunohistochemical expression in chromophobe carcinoma, eosinophilic variant. E, negative immunostain for parvalbumin in conventional (clear cell) carcinoma. F, negative immunostain for parvalbumin in papillary renal cell carcinoma. G, 1 of the 11 renal oncocytomas showing a granular cytoplasmic and nuclear parvalbumin expression. H, a case of oncocytoma completely negative for parvalbumin.
Oncocytomas consisted of a uniform population of cells with granular eosinophilic cytoplasm arranged in a solid alveoli in 12 cases and tubulocystic pattern in four cases.
In 10 different cases, specimens of metastatic lesion were also available. These included eight metastases of conventional (clear cell) carcinomas (three adrenal, one lung, and four pancreatic metastases), one lymph node metastasis of papillary renal cell carcinoma, and one pancreatic metastasis of chromophobe carcinoma (Fig. 3A).
The resected specimens containing the tumors had been fixed with buffered formalin and embedded in paraffin.
For all tumors, serial 5-μm sections were stained with hematoxylin and eosin, with Hale's colloidal iron technique (12) or immunostaining with anti-parvalbumin monoclonal antibody (clone PA-235, SIGMA Chemical, St. Louis, MO; dilution, 1:1000), anti-calbindin-D28K monoclonal antibody (Swant, Bellinzona, Switzerland; dilution, 1:200) and rabbit anti-calretinin antibody (Swant; dilution, 1:2000). To characterize the mitochondrial content of renal neoplasms, we used an anti-mitochondrial monoclonal antibody (clone 113-1, BIOGENEX, S. Ramon, CA; dilution, 1:1600). All the immunoreactions were developed using Envision peroxidase detection system (DAKO, Carpinteria, CA), a very sensitive method that also prevents possible false-positive staining due to endogenous biotin present in the tissue. Ten parvalbumin immunoreactions were also developed using the labeled polymer-AP DAKO Envision system in five chromophobe carcinomas and in five oncocytomas, respectively. No antigen-retrieval methods were used.
External positive and negative controls were provided by a series of tissues: 10 normal, 10 hyperplastic, and 10 adenomatous parathyroid glands; 10 normal and 10 adenomatous adrenal glands; 5 normal pancreas and 5 pancreatic endocrine tumors; 5 oncocytic adenomas; and 3 oncocytic carcinomas of the thyroid gland, mesothelial, and parenchymal components in normal lung tissue and two pulmonary carcinoids, and lymphoid tissues.
RESULTS
Histochemical Findings
All 32 chromophobe carcinomas stained with Hale's colloidal iron histochemical method. The staining intensity in most cases was high in Type 3, weak in Type 1, and intermediate in Type 2 cells. Oncocytomas, conventional (clear cell) renal carcinomas, and papillary renal cell carcinomas showed only focal and dust-like granular or luminal and coarse staining. The coarse and granular staining of hemosiderin was considered not specific.
Immunohistochemical Findings
Normal Fetal and Adult Kidney
The three calcium-binding proteins, parvalbumin, calbindin-D28K, and calretinin, were heterogenously expressed in normal fetal and adult renal samples.
In the normal fetal kidney, parvalbumin immunoreactivity was limited to the collecting ducts (Fig. 1, A–B); calbindin-D28K was strongly immunoreactive in the distal tubule, whereas calretinin was variably found in this segment of the distal nephron.
Strong parvalbumin immunostain in the collecting duct of the fetal renal parenchyma (A, B). Parvalbumin immunoreactivity in the cortex; the distal nephron is strongly positive, whereas the proximal nephron is negative (C); parvalbumin immunostains the distal tubule; the glomerulus and the proximal tubule are negative (D); parvalbumin immunoreactivity in the medulla of the normal adult kidney (E); strong expression of parvalbumin in a subset of cells of collecting duct, likely the intercalated cells (F).
In the adult kidney, parvalbumin and calbindin-D28K expressions overlapped. These molecules were constantly and strongly present in both the cytoplasm and nuclei of the distal convoluted tubules, connecting tubules (Fig. 1, C–D), and in a subset of collecting duct cells, likely intercalated cells (Fig. 1, E–F) By contrast, calretinin was weakly expressed in all the nephron segments.
Renal Tumors
The results describing the immunoreactivity of all the three calcium-binding proteins and the antimitochondrial antibody (113-1) on the different renal tumors are summarized in Table 1. The expression of the parvalbumin and antimitochondrial antibodies was semiquantitatively graded in neoplastic samples on the basis of both percentage of positively staining cells (0%, Grade 0; <60%, Grade 1; >60%, Grade 2) and intensity of the immunostaining (negative, −; weak, +; strong, ++) and summarized in Table 2.
All 32 chromophobe carcinomas were positive for parvalbumin, 30 displaying a strong cytoplasmic and nuclear immunoreactivity in the majority of cells, variable from 60 to 90%. In only two cases, the percentage of positive cells was 40%. The immunoreactivity in the cytoplasm was granular with a marked peripheral accentuation in Type 3 cells (Fig. 2B); Type 2 and 1 cells showed more prominent and coarse granular positivity of the entire cytoplasm (Fig. 2D), with the exception of the perinuclear halo of the latter type. The pancreatic metastasis of chromophobe carcinoma was also strongly and diffusely positive (Fig 3B). None of the primary and metastatic conventional (clear cell) renal carcinoma was positive for parvalbumin (Fig. 2E). Only scattered cells were parvalbumin positive in two of the papillary renal cell carcinomas (Fig. 2F), whereas the available lymph node metastasis of this tumor type was negative. Eleven of the 16 oncocytomas showed a variable granular cytoplasmic and nuclear parvalbumin expression in a proportion of neoplastic cells variable from 10 to 80%, whereas five cases were completely negative (Fig. 2, G–H). The pattern of positivity was not uniform in a positive oncocytoma; some areas displayed diffuse immunoreactivity with most cells staining positive, whereas other areas had only a few positively staining cells.
Calbindin-D28K was negative in all tumor types, whereas calretinin displayed a weak granular cytoplasmic immunoreactivity in two chromophobe renal carcinomas, three oncocytomas, two papillary renal cell, and one conventional (clear cell) carcinomas.
Finally, all tumor types were immunoreactive with the anti-mitochondrial antibody, with different cytoplasmic staining and intensity patterns. Chromophobe renal carcinoma showed a peripheral accentuation of the cytoplasmic granular immunoreactivity (Fig. 4A), oncocytomas, a very strong, diffuse and granular positivity (Fig. 4B), and conventional (clear cell) and papillary renal cell carcinomas, an irregular cytoplasmic staining (Fig. 4C–D). The granular cytoplasmic positivity was prevalently fine in oncocytomas and coarse in the carcinomas, but in some chromophobe carcinomas, a diffuse, fine-granular immunoreactivity distribution was also observed.
External Controls
All normal, hyperplastic, and adenomatous parathyroid glands were strongly and diffusely immunoreactive for parvalbumin but negative for calbindin-D28K and calretinin. Normal and adenomatous adrenal glands, normal pancreas and pancreatic endocrine tumors, oncocytic adenomas and carcinomas of the thyroid gland, mesothelial and parenchymal components in normal lung tissue, and pulmonary carcinoids and lymphoid tissues showed negative immunostaining for all three markers.
DISCUSSION
Our study shows that 1) parvalbumin is characteristically expressed by chromophobe renal carcinoma and its immunodetection is useful in distinguishing primary and metastatic chromophobe renal carcinoma from papillary renal cell and conventional (clear cell) carcinomas; 2) calbindin-D28K and calretinin immunostains were of no value for the purpose of the differential diagnosis of renal epithelial tumors; 3) about 70% of the oncocytomas also stained for parvalbumin, this suggesting a certain degree of morphological overlap between chromophobe carcinoma and this tumor type; and 4) parvalbumin is expressed in the collecting ducts of the fetal and adult kidney, this giving further support to the evidence that chromophobe carcinoma exhibits differentiation toward the phenotype of the intercalated cell of collecting ducts.
Although adequate sampling and a good understanding of the morphological features observed in the renal epithelial tumors usually minimize errors in their recognition and diagnosis, a certain degree of morphologic overlap exists between all renal tumor types. In particular, chromophobe carcinoma enters into the differential diagnosis with both conventional (clear cell) and papillary renal cell carcinomas, as well as oncocytoma, setting a clinically relevant dilemma (3). For this reason, the implementation of morphological analysis with histochemical and immunohistochemical stains has been attempted by various authors (10, 11, 12, 13, 14). However, the number of useful stains is limited, and the only helpful immunohistochemical finding consistently reported on chromophobe carcinoma is the absence of staining for vimentin (5, 14, 24).
Our data suggest that parvalbumin immunostain is useful in distinguishing chromophobe carcinoma from papillary renal cell and conventional (clear cell) carcinomas and seems more reliable than the anti-mitochondrial antibody 113-1 and colloidal iron stains. In facts, all of the primary and metastatic chromophobe carcinomas expressed strongly parvalbumin, whereas the primary and metastatic papillary renal cell and conventional (clear cell) carcinomas were negative in both the clear cell and granular cell components. Moreover, this immunostain was much simpler and more reproducible than iron colloidal stain and anti-mitochondrial immunostain. Calbindin-D28K and calretinin do not aid in differential diagnosis, as calbindin-D28K was completely negative in all tumors and calretinin showed a variable, weak, and poorly reproducible immunoreactivity in a minority of samples.
Parvalbumin immunostain was unable to provide a sharp distinction between oncocytoma and chromophobe carcinoma. However, the expression of parvalbumin in 70% of renal oncocytomas should not be surprising in the light of the recent morphologic (11, 25), ultrastructural (13, 26, 27), histochemical (13), and genetic (28) findings suggesting common features for both chromophobe carcinomas and oncocytomas from the distal nephron. Moreover, the absence of parvalbumin expression in the 30% of the oncocytomas may suggest the existence of differences in this group of tumors as also supported by genetic findings (29).
We report here that parvalbumin is selectively expressed in the collecting ducts of fetal and in the distal nephron of adult kidney, the latter reminding of the pattern described in adult rat kidney (16). Our finding further supports the hypothesis of a chromophobe neoplastic cells differentiation toward the phenotype of the intercalated cells of the collecting duct, which is based on the ultrastructural finding of cytoplasmic microvesicles and the characteristic expression of carbonic anhydrases C both in these cells and chromophobe carcinoma (7). Interestingly, the overlap of parvalbumin expression between chromophobe carcinoma and oncocytoma parallels the findings for carbonic anhydrases C immunoreactivity (7, 30).
Parvalbumin is one of the best-known cytosolic calcium–binding proteins able to modulate the levels of intracellular calcium. It can act as a cytosolic calcium ion buffer, but a possible indirect role in signal transduction by modifying calcium concentration has been proposed (31). The distribution of parvalbumin in the kidney matches fairly well that of calcium receptors in the distal tubule and in the proximal collecting duct, where the fine regulation of calcium readsorption takes place (32, 33). It is of interest that parathyroid glands possess the same calcium receptor found in the kidney, and a recent report has shown that parvalbumin, but not calbindin-D28K and calretinin, is expressed in parathyroid cells (31). The characteristic presence of parvalbumin in both chromophobe carcinoma and parathyroid cells raises the questions of its potential role as a modulator of calcium signals within chromophobe neoplastic cells of the distal nephron involved in calcium homeostasis.
In summary, our data support the utility of parvalbumin immunostain as a diagnostic tool for differential diagnosis of renal epithelial tumors and the hypothesis of a collecting-duct intercalated cell differentiation for chromophobe renal carcinoma.
References
Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, et al. The Heidelberg classification of renal cell tumours. J Pathol 1997; 183: 131–133.
Storkel S, Eble JN, Adlakha K, Amin M, Blute ML, Bostwick DG, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997; 80: 987–989.
Bonsib SM . Risk and prognosis in renal neoplasms. A pathologist's prospective. Urol Clin North Am 1999; 26: 643–660.
Thoenes W, Storkel S, Rumpelt HJ . Human chromophobe cell renal carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol 1985; 48: 207–217.
Thoenes W, Storkel S, Rumpelt HJ, Moll R, Baum HP, Werner S . Chromophobe cell renal carcinoma and its variants—a report on 32 cases. J Pathol 1988; 155: 277–287.
Crotty TB, Farrow GM, Lieber MM . Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol 1995; 154: 964–967.
Storkel S, Steart PV, Drenckhahn D, Thoenes W . The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol 1989; 56: 237–245.
Schwerdtle RF, Storkel S, Neuhaus C, Brauch H, Weidt E, Brenner W, et al. Allelic losses at chromosomes 1p, 2p, 6p, 10p, 13q, 17p, and 21q significantly correlate with the chromophobe subtype of renal cell carcinoma. Cancer Res 1996; 56: 2927–2930.
Bugert P, Gaul C, Weber K, Herbers J, Akhtar M, Ljungberg B, et al. Specific genetic changes of diagnostic importance in chromophobe renal cell carcinomas. Lab Invest 1997; 76: 203–208.
Cochand-Priollet B, Molinie V, Bougaran J, Bouvier R, Dauge-Geffroy MC, Deslignieres S, et al. Renal chromophobe cell carcinoma and oncocytoma. A comparative morphologic, histochemical, and immunohistochemical study of 124 cases. Arch Pathol Lab Med 1997; 121: 1081–1086.
Tickoo SK, Amin MB, Linden MD, Lee MW, Zarbo RJ . Antimitochondrial antibody (113-1) in the differential diagnosis of granular renal cell tumors. Am J Surg Pathol 1997; 21: 922–930.
Tickoo SK, Amin MB, Zarbo RJ . Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and pattern of staining. Am J Surg Pathol 1998; 22: 419–424.
Latham B, Dickersin GR, Oliva E . Subtypes of chromophobe cell renal carcinoma: an ultrastructural and histochemical study of 13 cases. Am J Surg Pathol 1999; 23: 530–535.
Taki A, Nakatani Y, Misugi K, Yao M, Nagashima Y . Chromophobe renal cell carcinoma: an immunohistochemical study of 21 Japanese cases. Mod Pathol 1999; 12: 310–317.
Reilly RF, Ellison DH . Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev 2000; 80: 277–313.
Bindels RJ, Hartog A, Timmermans JA, van Os CH . Immunocytochemical localization of calbindin-D28k, calbindin-D9k and parvalbumin in rat kidney. Contrib Nephrol 1991; 91: 7–13.
Zhu YY, Takashi M, Miyake K, Kato K . Sensitive enzyme immunoassay for human 28 kDa calbindin-D. Clin Chim Acta 1991; 201: 183–192.
Andressen C, Gotzos V, Berchtold MW, Pauls TL, Schwaller B, Fellay B, et al. Changes in shape and motility of cells transfected with parvalbumin cDNA. Exp Cell Res 1995; 219: 420–426.
Moutairou K, Hayez N, Pohl V, Pattyn G, Pochet R . Calbindin localization in African giant rat kidney (Cricetomys gambianus). Biochim Biophys Acta 1996; 1313: 187–193.
Niki I, Yokokura H, Sudo T, Kato M, Hidaka H . Ca2+ signaling and intracellular Ca2+ binding proteins. J Biochem (Tokyo) 1996; 120: 685–698.
Tos AP, Doglioni C . Calretinin: a novel tool for diagnostic immunohistochemistry. Adv Anat Pathol 1998; 5: 61–66.
Fuhrman SA, Lasky LC, Limas C . Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–663.
Akhtar M, Kardar H, Linjawi T, McClintock J, Ali MA . Chromophobe cell carcinoma of the kidney. A clinicopathologic study of 21 cases. Am J Surg Pathol 1995; 19: 1245–1256.
Avery AK, Beckstead J, Renshaw AA, Corless CL . Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol 2000; 24: 203–210.
Tickoo SK, Reuter VE, Amin MB, Srigley JR, Epstein JI, Mim KW, et al. Renal oncocytosis: a morphologic study of fourteen cases. Am J Surg Pathol 1999; 23: 1094–1101.
Erlandson RA, Shek TW, Reuter VE . Diagnostic significance of mitochondria in four types of renal epithelial neoplasms: an ultrastructural study of 60 tumors. Ultrastruct Pathol 1997; 21: 409–417.
Tickoo SK, Lee MW, Eble JN, Amin M, Christopherson T, Zarbo RJ, et al. Ultrastructural observations on mitochondria and microvesicles in renal oncocytoma, chromophobe renal cell carcinoma, and eosinophilic variant of conventional (clear cell) renal cell carcinoma. Am J Surg Pathol 2000; 24: 1247–1256.
Dijkhuizen T, van den Berg E, Storkel S, de Vries B, van der Veen AY, Wilbrink M, et al. Renal oncocytoma with t(5;12;11), der(1;8) and add(19): “true” oncocytoma or chromophobe adenoma? Int J Cancer 1997; 73: 521–524.
Zambrano NR, Lubensky IA, Merino MJ, Linehan WM, Walther MM . Histopathology and molecular genetics of renal tumors toward unification of a classification system. J Urol 1999; 162: 1246–1258.
Storkel S, Pannen B, Thoenes W, Steart PV, Wagner S, Drenckhahn D . Intercalated cells as a probable source for the development of renal oncocytoma. Virchows Arch B Cell Pathol Incl Mol Pathol 1988; 56: 185–189.
Pauls TL, Portis F, Macri E, Belser B, Heitz P, Doglioni C, et al. Parvalbumin is expressed in normal and pathological human parathyroid glands. J Histochem Cytochem 2000; 48: 105–111.
Schneeberger PR, Heizmann CW . Parvalbumin in rat kidney. Purification and localization. FEBS Lett 1986; 201: 51–56.
Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC . Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci U S A 1995; 92: 131–135.
Acknowledgements
This study was supported by grants from Fondazione Cassa di Risparmio di Verona, Consorzio Studi Universitari di Verona, Associazione Italiana Ricerca Cancro (to MC), and Ricerca Sanitaria Finalizzata-Regione Veneto, Italy.
The authors thank Dr. Paola Capelli for helpful discussion and Arianna Bellotti, Licia Montagna, Paola Piccoli, and Giorgio Bettio for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martignoni, G., Pea, M., Chilosi, M. et al. Parvalbumin Is Constantly Expressed in Chromophobe Renal Carcinoma. Mod Pathol 14, 760–767 (2001). https://doi.org/10.1038/modpathol.3880386
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880386
Keywords
This article is cited by
-
TSC loss is a clonal event in eosinophilic solid and cystic renal cell carcinoma: a multiregional tumor sampling study
Modern Pathology (2022)
-
Parvalbumin immunohistochemical expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney
Virchows Archiv (2021)
-
Biomarkers of Renal Tumors: the Current State and Clinical Perspectives
Current Urology Reports (2017)
-
Value of PAX8, PAX2, napsin A, carbonic anhydrase IX, and claudin-4 immunostaining in distinguishing pleural epithelioid mesothelioma from metastatic renal cell carcinoma
Modern Pathology (2013)
-
Immunohistochemical marker panel differentiates between the three most common subtypes of renal cell carcinoma independent from histomorphologic criteria
Virchows Archiv (2012)