Abstract
A full-term, healthy male neonate was delivered by caesarian section to a 26-year-old primigravida woman who had a history of fever and upper respiratory tract infection. On the fourth day of life, the neonate developed a sepsis-like syndrome, acute respiratory and renal failure, and disseminated intravascular coagulopathy. He died 13 days after birth. Postmortem examination revealed jaundice, anasarca, massive hepatic necrosis, adrenal hemorrhagic necrosis, renal medullary hemorrhage, hemorrhagic noninflammatory pneumonia, and severe encephalomalacia. Echovirus type 6 was isolated from blood, liver, and lungs. Although uncommon, echovirus type 6 infection may produce a spectrum of pathologic findings similar to those seen with the more commonly virulent echovirus type 11.
Similar content being viewed by others
INTRODUCTION
Echoviruses are single-stranded RNA viruses of the genus Enterovirus of the family Picornaviridae that may occasionally cause overwhelming disease and death in neonates (1, 2). Of the 31 types of echoviruses, type 11 is the most frequent cause of serious neonatal morbidity and mortality, often presenting as fulminant hepatitis, infection of the central nervous system, or both (3). Echovirus type 6 infection is an uncommon cause of neonatal mortality, with only a few reported cases of severe or fatal neonatal infection (3, 4, 5, 6, 7). We present the case of a newborn infant with fatal echovirus 6 infection and describe the unusual pathologic findings. We also review the literature about severe neonatal echovirus 6 infections.
CASE REPORT
A full-term boy appropriate for his gestational age was born via caesarian section to a 26-year-old G1P0 Latin American woman who had a medical history of a well-controlled seizure disorder for which she was receiving carbamazepine. She had received regular prenatal care, with negative prenatal serologic tests for human immunodeficiency virus, hepatitis B virus, and syphilis. Two weeks before delivery, she experienced fever and an upper respiratory tract infection. At birth, the infant weighed 3838 g and had an Apgar score of 9. The neonate was put under an oxygen hood, then was later slowly weaned from it. On his fourth day of life, the neonate developed fever (38.6°C) and was observed to have decreased activity. An area of hyperemia and swelling was seen on the right shoulder. This area of swelling and redness increased in size during the next few days. He received ampicillin, gentamicin, ceftazidime, and acyclovir after blood was collected for viral and bacterial cultures. Cranial ultrasonography results were normal.
On the fifth day of life, the neonate experienced respiratory difficulty, which required intubation and mechanical ventilation. He remained febrile and developed oliguria that later progressed to renal insufficiency. Sepsis and disseminated intravascular coagulopathy were suspected, and blood and blood products were provided. On the ninth day of life, he suffered cardiopulmonary arrest twice and remained neurologically compromised thereafter. He was transferred to the University of Texas Medical Branch at Galveston on his 12th day of life for possible hemodialysis. Clinical diagnoses at admission included sepsis, respiratory failure, and renal failure. Cranial ultrasonography at this time showed periventricular leukomalacia, edema, and intracranial hemorrhage. His condition continued to deteriorate, and he died on his 13th hospital day. A complete autopsy was performed 18 hours after death.
Autopsy
External examination of the body revealed an extremely edematous and jaundiced infant with no dysmorphic features or congenital anomalies. A 7- by 7-cm violaceous cutaneous area with a central 1- by 2-cm ulcer was present on the right shoulder, and multiple ecchymoses were observed on both upper extremities.
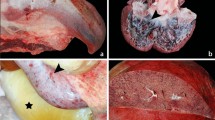
Petechiae were present in the epicardium, subendocardium, and thymic surface. The thymus was involuted (weight, 1.2 g; expected weight, 11.5 ± 3.7 g). The liver appeared mottled, with pale, punctate areas surrounded by hyperemic parenchyma (Fig. 1A). Patches of the duodenum and ileum revealed dark red, granular mucosa but no gross intraluminal hemorrhage (Fig. 1B). The remainder of the gastrointestinal mucosa was hyperemic. The kidneys were firm and dark brown, with blood-red medullae. The spleen was dark red and soft. The adrenal glands and periadrenal fat were markedly hemorrhagic (Fig. 1C). The brain was extremely macerated, fragmented, and autolyzed. Subarachnoid hemorrhage and intraparenchymal cerebral hemorrhage were noted. Mild lymphocytic meningitis was also identified.
A, gross photograph of liver shows bright red parenchyma corresponding to congestion and hemorrhage into extensive regions of coagulative necrosis. B, gross photograph of small intestine showing extensive hemorrhagic necrosis of the mucosa. C, gross photograph of cross sections of adrenal glands shows hemorrhage largely replacing the cortex and medulla. D, photomicrograph of massive hepatic necrosis with dystrophic calcification (arrowhead) and preserved viable cells in portal triads and periportal hepatic parenchyma. Hematoxylin and eosin; original magnification, 50×. E, photomicrograph of lung with alveolar hemorrhage, bronchial and bronchiolar fibrinous exudates, and interstitial pneumonia. The adjacent lobule (lower left) shows only alveolar fibrin and interstitial pneumonia. Hematoxylin and eosin; original magnification, 100×. F, photomicrograph of severe hemorrhagic necrosis of adrenal zonae fasciculata and reticularis and medulla with partial preservation of the zona glomerulosa. Hematoxylin and eosin; original magnification, 50×.
Microscopic examination of the liver demonstrated massive and diffuse coagulative necrosis with multiple foci of dystrophic calcification of necrotic hepatocytes (Fig. 1D). The lungs contained multifocal pulmonary intra-alveolar hemorrhage, hyaline membranes, moderate lymphocytic and macrophage infiltrate, and fibrinous exudates (Fig. 1E). There was extensive renal medullary hemorrhage, adrenal hemorrhage, and necrosis (Fig. 1F). No viral inclusions were detected.
Microbiology Results
Postmortem samples of blood, lung, and liver inoculated into Rhesus monkey kidney cell tube cultures (BioWhittaker Inc., Walkersville, MD) yielded cytopathic effect characteristic of enterovirus on 2, 5, and 5 days after inoculation, respectively. Screening with pan-serotype monoclonal antibody mixtures (Chemicon International Inc., Temecula, CA) identified the isolate as an echovirus. Final serotyping was performing by the Texas Department of Health Bureau of Laboratories, Austin, and identified the isolate as echovirus 6.
DISCUSSION
Of the more than 30 echovirus types, echovirus 11 has been reported to be the most common cause of serious neonatal infection (3, 8, 9), usually presenting as hepatitis or central nervous system infection. The reason for the relatively greater virulence of this echovirus type compared with that of other types is unclear (10). Other echovirus types that have been documented to occasionally cause fatal infection include types 7 (11), 9 (12), 14 (13), and 19 (3, 14, 15). Echovirus 6, however, has only rarely been documented as the cause of serious morbidity and mortality. A review of the English-language scientific literature yielded only five reported cases of fatal echovirus 6 infections (3, 4, 5, 6) (Table 1).
We report an infant infected with echovirus 6 who presented with shock, disseminated intravascular coagulopathy, and renal failure. Necropsy revealed hepatic necrosis, renal hemorrhage, adrenal hemorrhagic necrosis, gastrointestinal hemorrhage, and severe encephalomalacia. This septic picture with hepatic necrosis and hemorrhage is reportedly the most common presentation of a severe echovirus infection (3). However, massive hepatic and adrenal necrosis has been reported mostly in newborns with echovirus 11 at autopsy (16) and is not a common observation in echovirus 6 infections. To our knowledge, only one case of hepatoadrenal necrosis in an echovirus 6 infection has been reported aside from our case (5).
The clinical differential diagnosis is that of neonatal sepsis and includes bacteremia caused by Escherichia coli or other gram-negative bacteria such as Pseudomonas aeruginosa or by Streptococcus agalactiae or other gram-positive bacteria such as Staphylococcus aureus. In addition to disseminated enteroviral infection caused by an echovirus or a coxsackievirus, disseminated herpesvirus or cytomegalovirus should be considered. The massive hepatic necrosis particularly suggests neonatal herpesvirus or echovirus infection. The hemorrhagic adrenal necrosis suggests bacterial sepsis with disseminated intravascular coagulation or neonatal herpesvirus or echovirus infection. Involvement of the leptomeninges can occur in any of the above infections. However, prominent gross hemorrhages in the renal medullae has been described as a hallmark of echovirus type 11 infection.
The ideal method for diagnosis of echovirus infections at autopsy is isolation and identification of the virus from affected organs in cell culture (17, 18, 19). A number of cell lines (RD cells, human rhabdomyosarcoma cells, green monkey kidney cells, HeLa cells, human embryonic lung fibroblasts, and primary monkey kidney cells) have been used routinely to isolate echoviruses. Although echoviruses are thermostable in the presence of divalent cations, are acid stable, and do not have a lipid envelope, viral infectivity is preserved for progressively longer periods during transport or storage at 4°C, −20°C, and −70°C than at room temperature and in the presence of antibiotics such as penicillin and streptomycin. In addition to tissue samples such as brain, lung, liver, lymph nodes, and heart muscle, other samples including feces, intestinal contents, intestinal wall, cerebrospinal fluid, cutaneous vesicle fluid, and pharyngeal swab may be collected for viral isolation. Because many echovirus infections are asymptomatic and fecal shedding may be prolonged for several weeks, the recovery of virus from only the feces should be interpreted cautiously.
Postmortem serologic diagnosis of neonatal echovirus infection is generally not useful because of the numerous serotypes, likely occurrence of death before a detectable humoral immune response occurs, and the presence of transplacentally transferred antibodies to many of the enteroviruses previously encountered by the mother (19).
Another useful diagnostic approach is reverse transcriptase–polymerase chain reaction directed against the highly conserved region at the 5′ end of the enteroviral genome (17, 18). Use of primers that amplify type-specific regions of the genome and sequencing of the amplified DNA products make possible the identification of the virus. Electron microscopy seldom detects virus at concentrations less than 107 particles per gram of tissue and does not distinguish among the various picornaviruses. Although numerous immunologic approaches to detection of enteroviruses have been described, none has achieved widespread, successful use.
Of the five cases of fatal echovirus 6 neonatal infections described in the literature, three patients represent illness with the entire course occurring during the first 2 weeks of life, whereas one patient presented with a later onset and a longer period of illness, reaching 2 months of age (3, 4, 5, 6) (Table 1). Two cases were associated with a maternal history of upper respiratory tract infection, and in both patients, delivery was performed by caesarian section (5, 6). This history of maternal illness and caesarian section delivery is similar to the patient we studied. Two of the patients reported in the literature were infants born to healthy mothers. The pathology of these five patients manifested as two general presentations: disseminated intravascular coagulopathy with hepatomegaly, and pneumonitis.
Two of the reported patients had disseminated intravascular coagulopathy with hepatomegaly, hepatitis, or both, whereas two patients had pneumonitis (4, 5, 6). One patient presented with hepatitis without disseminated intravascular coagulopathy (3). Our patient was characterized by multiorgan necrosis and hemorrhage consistent with disseminated intravascular coagulopathy and prominent hepatic and adrenal necrosis, similar to the observations made by Carolane et al. (4) and Krous et al. (5). One case of fatal pneumonia revealed pulmonary interstitial fibrosis, septal inflammation, and poorly formed hyaline membranes at necropsy (6). The possibility that these changes may have been attributable to hyperbaric oxygenation of the newborn, however, could not be excluded (6). In the patient we studied, hyaline membranes, lymphohistiocytic inflammation, and hemorrhage were observed without fibrosis.
In a study of 61 cases of perinatal echovirus infection, Modlin (3) reported only a single case caused by echovirus 6. This solitary patient with echovirus 6 presented with hepatitis but without hepatoadrenal necrosis. Among neonatal echovirus infections, presentation with hepatitis is reported to be highly associated with a greater mortality (3). From other studies, echovirus 6 has also been shown to present as herpes zoster–like vesiculo-bullous eruptions, meningitis, or orchitis (20, 21, 22, 23).
Fatal echovirus is rare in adults and immunocompetent children. It is postulated that neonates are more susceptible to this infection, presumably because of relative immunodeficiency and a lack of transplacentally acquired neutralizing antibody to the particular echovirus type. Male infants and premature neonates are more susceptible to echovirus disease, although the precise reasons behind these observations are not known (3).
Vertical transmission from the mother to infant and nosocomial infections from hospital personnel are the two major means of transmission to neonates. Transplacental transmission has been suggested as a mode of transmission in neonatal echovirus 11 infections (8). Echovirus 11 has been isolated from amniotic fluid in a stillborn baby (24) and from the cord blood of a neonate at birth (25), supporting this theory. A transplacental route has also been suggested for echoviruses 14 and 19 (13, 14). In Modlin's review (3), approximately 70% of the cases of echovirus infection were associated with an acute maternal illness during the last 2 weeks of pregnancy, suggesting vertical transmission from mother to child in the perinatal period. On the other hand, about 18% of the echovirus infections were thought to have been acquired in the postpartum period from hospital personnel by direct contact with the neonates. In these cases, there was no maternal history of illness, and interestingly, the onset of illness appeared to be much later than in those that were thought to be vertically acquired (about 10 to 14 d versus 1 to 7 d postpartum). In addition, infants with nosocomial echovirus infections developed severe hepatitis less often than the other infants, resulting in a lower mortality rate.
Among the reported patients who died of echovirus 6, including the patient we studied, hepatic necrosis and early neonatal death were seen in four patients, the exception being one neonate who died at 2 months of age. In the patient we studied, the mother's history of fever and an upper respiratory tract infection 2 weeks before delivery suggest a transplacental mode of virus transmission. The caesarian mode of delivery rules out the possibility of infection from perineal fecal contamination or cervicovaginal sources.
In summary, a case of fatal neonatal echovirus 6 infection presented with shock, subtotal hepatic necrosis, severe encephalomalacia, hemorrhagic pneumonitis, and renal and adrenal hemorrhagic necrosis. This report illustrates that echovirus 6, although an uncommon cause of serious neonatal disease, can produce a spectrum of changes similar to that seen with the more commonly virulent echovirus type 11.
References
Modlin JF . Picornavirididae. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 1888–95.
John TJ, Walker DH . Enterovirus infections, including poliomyelitis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens, and practice. Philadelphia: Churchill Livingston; 1999. p. 1123–32.
Modlin JF . Perinatal echovirus infection: insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis 1986; 8: 918–26.
Carolane DJ, Long AM, McKeever PA, Hobbs SJ, Roome AP . Prevention of spread of echovirus 6 in a special care baby unit. Arch Dis Child 1985; 60: 674–6.
Krous HF, Dietzman D, Ray CG . Fatal infections with echovirus types 6 and 11 in early infancy. Am J Dis Child 1973; 126: 842–6.
Boyd MT, Jordan SW, David LE . Fatal pneumonitis from congenital echovirus type 6 infection. Pediatr Infect Dis J 1987; 6: 1138–9.
Blokziji ML, Koskiniemi M . Echovirus 6 encephalitis in preterm baby. Lancet, 2: 164–5, 1989.
Modlin JF . Fatal echovirus 11 disease in premature neonates. Pediatrics 1980; 66: 775–80.
Berry PJ, Nagington J . Fatal infection with echovirus 11. Arch Dis Child 1982; 57: 22–9.
Hill WMJ . Are echovirus still orphans? Br J Biomed Sci 1996; 53: 221–6.
Ho-Yen DO, Hardie R, McClure J, Cunningham NE, Bell EJ . Fatal outcome of echovirus 7 infection. Scand J Infect Dis 1989; 21: 459–61.
Rawls WD, Shorter RG, Herrmann EC . Fatal neonatal illness associated with ECHO 9 virus. Pediatrics 1964; 33: 278–9.
Hughes JR, Wilfert CM, Moore M, Benirschke K, de Hoyos-Guervara E . Echovirus 14 infections associated with fatal neonatal hepatic necrosis. Am J Dis Child 1972; 123: 61–7.
Philip AGS, Larson RJ . Overwhelming neonatal infection with ECHO 19 virus. J Pediatr 1973; 82: 391–7.
Arnon R, Naor N, Davidson S, Katz K, Mor C . Fatal outcome of neonatal echovirus 19 infection. Pediatr Infect Dis J 1991; 10: 788–9.
Mostoufizadeh M, Lack EE, Gang DL, Perez-Atayde AR, Driscoll SG . Postmortem manifestations of echovirus 11 sepsis in five newborn infants. Hum Pathol 1983; 4: 818–23.
Grandien M, Forsgren M, Ehrnst A . Enteroviruses. In: Lennette EH, Lennette DA, Lennette ET, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 7th ed. Washington, DC: American Public Health Association; 1995. p. 279–97.
Rotbart HA . Enteroviruses. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 7th ed. Washington, DC: ASM Press; 1999. p. 990–8.
Melnick JL . Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 3rd ed. Philadelphia: Lippincott-Raven, 1996. p. 655–711.
Meade RH, Chang TW . Zoster-like eruption due to echovirus 6. Am J Dis Child 1979; 133: 283–4.
Foreman RE, Guiterrez A . Aseptic meningitis associated with echovirus type 6 and 9 infections in Carlsbad, New Mexico. Rocky Mountain Med J 1978; 75: 209–13.
Ashwell MJ, Smith DW, Phillips PA, Rouse IL . Viral meningitis due to echovirus types 6 and 9: epidemiological data from Western Australia. Epidemiol Infect 1996; 117: 507–12.
Welliver RC, Cherry JD . Aseptic meningitis and orchitis associated with echovirus 6 infection. J Pediatr 1978; 92: 239–40.
Skeels MR, Williams JJ, Ricker FM . Perinatal echovirus infection. N Engl J Med 1981; 305: 1529–30.
Jones MJ, Kolb M, Votava HJ, Johnson RL, Smith TF . Intrauterine echovirus type 11 infection. Mayo Clin Proc 1980; 55: 509–12.
Acknowledgements
The authors thank Kelly Cassity for expert secretarial assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ventura, K., Hawkins, H., Smith, M. et al. Fatal Neonatal Echovirus 6 Infection: Autopsy Case Report and Review of the Literature. Mod Pathol 14, 85–90 (2001). https://doi.org/10.1038/modpathol.3880260
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880260
Keywords
This article is cited by
-
Clinical characteristics of severe neonatal enterovirus infection: a systematic review
BMC Pediatrics (2021)
-
Molecular epidemiological characteristics of echovirus 6 in mainland China: extensive circulation of genotype F from 2007 to 2018
Archives of Virology (2021)
-
Reliability of non-culturable virus monitoring by PCR-based detection methods in environmental waters containing various concentrations of target RNA
Journal of Microbiology (2012)
-
Minimum Infective Dose of the Major Human Respiratory and Enteric Viruses Transmitted Through Food and the Environment
Food and Environmental Virology (2011)
-
Molecular epidemiology of Echovirus 6 in Greece
European Journal of Clinical Microbiology & Infectious Diseases (2009)