Abstract
Cyclins D1 (cD1) and E (cE) are G1 phase cyclins believed to participate in the pathogenesis of malignancy. Overexpression of cD1 has been reported to influence prognosis in squamous cell carcinomas (SCC) of the larynx, but was not significant in a limited study of non-small cell lung cancers (NSCLC). Altered expression of cE has been proposed as another potential prognostic marker in malignancy but its possible role in NSCLC has not been elucidated. In order to determine the prognostic value of cD1 and cE in NSCLC, paraffin-embedded sections of 467 NSCLC were immunostained with monoclonal antibody to cD1 (1:500, PharMingen, San Diego, CA) and 400 NSCLC with MA to cE (1:2500, PharMingen) using an enhanced sensitivity avidin-biotin complex technique. The number of tumor cells with nuclear and/or cytoplasmic immunopositivity was graded on a scale of: 0 = less than 1%, 1 = 1 to 10%, 2 = 10 to 25%, 3 = 25 to 50%, 4 = 50 to 75%, 5 = more than 75%. Results were correlated with survival by Kaplan-Meier survival plot using Stat-View software (Abacus Concepts, Berkeley, CA). Overall, 426 NSCLC with cD1 and 360 NSCLC with cE had adequate follow-up (median, 76 mo) for survival analysis. Both cyclins independently showed significance in prognosis of SCC but not other cell types. For cD1, absence of immunostaining was associated with worse prognosis than any immunopositivity for all stages of SCC (P = .025). For cE, Stage I and II SCC with less than 50% immunopositivity had a worse prognosis (P = .029). Of 70 Stage I and II SCC immunostained for both monoclonal antibodies, 55% of patients with tumors that demonstrated both absence of cD1 staining and cE immunopositivity in less than 50% of cells were dead at 5 years compared to 35% of patients with tumors that demonstrated positive staining with cD1 and cE immunopositivity in more than 50% of cells. These results strongly suggest cD1 and cE can independently predict prognosis in early stage SCC. Worse prognosis was associated with loss of expression, consistent with mechanisms other than overexpression of these cyclins in the progression of SCC.
Similar content being viewed by others
INTRODUCTION
Lung cancer is now the leading cause of cancer deaths in both men and women in the United States (1). Histologically, lung cancer can be classified into one of four major subtypes: adenocarcinoma (AC), squamous cell carcinoma (SCC), large cell carcinoma (LC), and small cell carcinoma. Whereas small cell lung cancers have a uniformly aggressive clinical picture, non-small cell carcinomas (NSCLC) are a heterogeneous group of tumors with variable clinical courses. Therefore, the detection of prognostic markers is essential in order to specify treatment protocols and determine which patients need more aggressive surgery, adjuvant chemotherapy, or radiotherapy.
Studies have reported alterations of proto-oncogenes and tumor suppressor genes that may contribute to the progression of NSCLC and serve as potential prognostic markers. Cyclins D1 and E are relatively recently described proteins implicated in the pathogenesis of NSCLC. Cyclins (primarily D1, D2, D3, and E) and their associated cyclin-dependent kinases regulate progression of the cell cycle through the G1 phase and into the S-phase of DNA replication (2). Overexpression of cD1 and cE has been demonstrated to shorten the G1 phase of the cell cycle (3, 4). Increased expression of cyclin D1 (cD1), as a result of amplifications and rearrangements, has been reported in parathyroid adenomas (5); some B-cell lymphomas (6); and in esophageal (7), head and neck (8, 9), hepatic (10, 11), colorectal (12), and some breast carcinomas (13, 14, 15). These studies have suggested that overexpression of cD1 shortens the G1 phase and accelerates cell growth, thus positively contributing to oncogenesis.
Studies examining the use of immunohistochemical staining for cD1 or cyclin E (cE) as a potential prognostic marker are extremely rare. Overexpression of cD1 has been reported to negatively influence prognosis in squamous cell carcinomas (SCC) of the larynx (8), but was of no prognostic value in a limited study of NSCLC (16) and mammary carcinomas (17). Increased expression of cE, another key regulatory component of cell cycle control, has been demonstrated in colorectal (18) and mammary carcinomas (19) and has been proposed as a potential and possibly better prognostic marker for breast cancer than cyclin D (19), but its possible role in NSCLC has not been elucidated. Therefore, this study was undertaken to determine the prognostic value of cD1 and cE in NSCLC.
MATERIALS AND METHODS
cD1 Patient and Specimen Selection
Paraffin blocks from wedge biopsies and resections (segmentectomies, lobectomies, pneumonectomies) of primary NSCLC for which patient follow-up was available were retrieved from the archives of The Methodist Hospital in Houston, Texas. A total of 467 specimens (5% wedge biopsies, 95% resections) with follow-up were available for study. The series included 270 AC, 130 SCC, and 67 LC. Pathology reports and cancer registry data were reviewed to determine tumor stage.
cD1 Immunohistochemical Staining
After antigen retrieval by microwave irradiation in citrate buffer, sections were incubated overnight with the antibody used for the detection of cD1 (purified mouse anti-human cD1 mAB, clone G124–326, 1:500, San Diego, CA). Detection was by Vectastain Elite mouse IgG immunoperoxidase kit (Vector, Burlingame, CA). Diaminobenzidine was used as the chromagen, and the sections were lightly counterstained with hematoxylin. With each batch of samples, a positive control (colon) was evaluated.
cE Patient and Specimen Selection
Paraffin blocks from wedge biopsies and resections (segmentectomies, lobectomies, pneumonectomies) of primary NSCLC for which patient follow-up was available were retrieved from the archives of The Methodist Hospital in Houston, Texas. A total of 400 specimens (6% wedge biopsies, 94% resections) with follow-up were available for study. The series included 232 AC, 113 SCC, and 55 LC. Pathology reports and cancer registry data were reviewed to determine tumor stage.
cE Immunohistochemical Staining
After antigen retrieval by microwave irradiation in citrate buffer, sections were incubated overnight with the antibody used for the detection of cE (purified mouse anti-human cE mAB, clone HE12, 1:2500, San Diego, CA). Detection was by Vectastain Elite mouse IgG immunoperoxidase kit (Vector). Diaminobenzidine was used as the chromagen, and the sections were lightly counterstained with hematoxylin. With each batch of samples, a positive control (spleen) was evaluated.
Grading and Statistical Analysis
The number of tumor cells with nuclear and/or cytoplasmic immunopositivity was graded on a scale of: 0 = less than 1%, 1 = 1 to 10%, 2 = 10 to 25%, 3 = 25 to 50%, 4 = 50 to 75%, 5 = more than 75%. Histologic subtype, stage, and percent of tumor cells staining positively were correlated with survival using the Kaplan-Meier method. Significance was tested for by the log-rank (Mantel-Cox) method, and P values less than .05 were considered statistically significant. Stat-View software from Abacus Inc. was used for all statistical analyses.
RESULTS
cD1 Staining
The patients ranged in age from 35 to 90 years at the time of diagnosis, with a median age of 64.6 years and a mean of 64.5 years. Lung cancer was known to be the primary cause of death in 88 patients. Thirty patients (7%) were nonsmokers. The male to female ratio was 263:163.
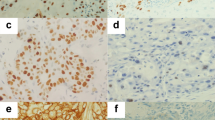
The cD1 cytoplasmic staining results are summarized in Table 1. Overall, 48% of tumors demonstrated positive staining. The data was further subdivided by histologic subtype, stage, and percent of tumor cells staining positively (Tables 1 and 2). The immunostaining pattern was predominantly cytoplasmic (Fig. 1); however, 29 tumors also demonstrated focal nuclear staining.
Four hundred twenty-six patients had adequate follow-up for examination by Kaplan-Meier survival analysis. For analysis purposes, follow-up was truncated at 4 years. Of the 426 patients, 96% were followed for at least 4 years. At 4 years, 214 patients were dead, 197 were alive, and 15 had been lost to follow-up. The only subset that generated a statistically significant P value was the group of all 130 SCC (all stages). This group showed a trend for better survival in cD1-positive versus negative tumors (P = .0902). When follow-up was truncated at 5 years, this value dropped to P = .0555; when follow-up was further censored to 4 years, this became statistically significant (P = .0246, Fig. 2). The same trend was observed regardless of stage of SCC. No correlation between cytoplasmic staining score and grade or stage was observed. In fact, it was relatively consistent across such categories. Analysis of the 29 tumors that showed focal nuclear staining did not demonstrate a clear relationship to cell type, stage, grade, cytoplasmic staining, smoking history, or prognosis.
cE Staining
The patients ranged in age from 39 to 90 years at the time of diagnosis, with both the median and mean age being 64.2 years. Lung cancer was known to be the primary cause of death in 88 patients. Twenty patients (5.5%) were nonsmokers. The male to female ratio was 230:130.
The overall cE staining results are summarized in Table 3. Overall, 32% of tumors demonstrated positive staining in more than half of the cells. The data was further subdivided by histologic subtype, stage, and percent of tumor cells staining positively. Table 4 outlines the cE immunostaining results for SCC. The cE immunostain showed intense nuclear staining; no cytoplasmic staining was demonstrated (Fig. 3).
Three hundred sixty cases had adequate follow-up for examination by Kaplan-Meier survival analysis. Follow-up was not truncated for this study set. Two hundred thirty-nine patients were followed until their death; the remaining 121 patients have been tracked to the present or have been lost to contact. The mean follow-up for these 121 patients was 82.6 mo, with a median of 76 mo. The only subset that generated a statistically significant P value was the group of all 70 stage I and II SCC. This group demonstrated significantly better survival in cases showing immunopositivity in greater than 50% of cells (P = .029, Fig. 4).
A seemingly contradictory trend was noted for cE positivity in 184 stage I and II AC (including bronchioloalveolar carcinomas). Comparing survival analysis for patients with negatively versus positively staining tumors, absence of staining showed a trend toward better survival (P = .0647). This P value is very close to statistical significance.
Conclusion
The results of our study strongly suggest that cD1 and cE expression is associated with better prognosis in a human cancer, specifically NSCLC. The expression of cD1 in our 130 pulmonary SCC demonstrates a trend toward increased patient survival outcome, which was statistically significant only at a relatively short (4 years) follow-up period. Long-term follow-up may achieve statistical significance when applied to a larger series of SCC cases. In early stage SCC, better survival was demonstrated in cases showing immunopositivity for cE in more than 50% of tumor cells. These results were statistically significant. The cD1 and cE expression provided prognostic significance in SCC, but not tumors of other cell types.
Most of the previous studies involving cD1 and cE have found overexpression of these cyclins in human tumors from various sites and suggested a link between overexpression and oncogenesis. Knowledge of the cell cycle provides a reasonable explanation to support these findings. The retinoblastoma-1 (Rb) tumor suppressor gene produces a protein (pRB) thought to be the major repressor of G1 phase progression (20). pRB is active in early G1 in its hypophosphorylated form; in mid/late G1, the protein becomes inactivated by phosphorylation. Cyclins D1 and E, in association with their catalytic partners, the cyclin-dependent kinases (cdk), are responsible for this phosphorylation of pRB and regulate progression of the cell cycle from G1 into S-phase (2). The protein product of Rb appears to inhibit cD1 and cE promotor activity by binding E2F transcription factors. In mid-to-late G1, when pRB is initially phosphorylated, perhaps by cdk-cyclin D complexes, E2F transcription factors are released, which promote transcription of the cD1 and cE genes (21, 22). Increased cD1 and cE expression continues pRB phosphorylation, releasing additional E2Fs that continue cD1 and cE transcription. Therefore, it is plausible that overexpression of cD1 or cE heightens phosphorylation of pRB and promotes cellular proliferation by suppressing pRB activity. This concept of cD1 and cE as positive regulators of cell proliferation is supported by studies that have demonstrated that microinjection of anti-cyclin D1 and E antibodies prevent S-phase entry (3, 23, 24). The studies that have found overexpression of cD1 or cE in tumors have postulated that cD1 and cE are positively involved in tumorigenesis because increased levels of cD1 and cE have been shown to promote cell growth by shortening the G1 phase (3, 4). It is possible then that overexpression of these cyclins may contribute not only to tumorigenesis but also to clinical aggressiveness of a tumor.
However, our study has shown that cD1 and cE immunopositivity confers a better prognosis in human lung cancers, and recent studies have raised the possibility that cD1 actually functions as a negative regulator of cellular proliferation (3, 4, 25). We have already established the feedback mechanism between cD1, cE, and pRB. Several studies have reported a lack of cD1 expression in pRB-deficient tumors (21, 26). The absence of cD1 in the higher stage tumors in our study may be related to mutations in the Rb-1 gene. It is likely that mutations in Rb-1 gene are more significant than those of the cyclins and further study involving cD1 and Rb-1 expression in concert is necessary to support this intriguing possibility. Additionally, this concept is supported by Lukas et al. (27), who reported that microinjection of anti-cyclin D1 antibodies accelerates S-phase entry in pRB-deficient mouse embryonic fibroblasts. The results of this enticing study are in accord with our findings that suggest lack of cD1 expression promotes cell proliferation.
Another possible explanation for our findings concerns the amount of cD1 and the timing at which these levels occur. It has been demonstrated that high levels of cD1 occurring at the G1/S phase junction arrest S-phase entry (3, 23, 28), suggesting that the amount and timing of cD1 can exert different effects on cellular proliferation. For unknown reasons, it appears that cD1 can be either a positive or negative regulator of cellular proliferation in different situations. Additionally, cD1 has been shown to accumulate in senescent, differentiated cells, which may explain why cD1 immunopositivity was present in the better-differentiated tumors in our study (29, 30). In summary, the potential oncogenic properties of cD1 in vivo are unclear.
Interestingly, our study reveals a seemingly contradictory trend for early stage AC. Tumors showing absence of staining for cE demonstrated a trend toward better survival. This is in agreement with other studies that have demonstrated increased cE expression in increasing grade and stage of AC of the breast (19).
The cyclins and pRB are only two components of a complex regulatory pathway. p16 is a member of a family of regulatory proteins that compete with cD1 for the binding of cdk 4 and 6 (20). Cellular growth factors determine the amount of cdk 4 or 6 associated with cD1 or p16, thereby determining phosphorylation of pRB and cell proliferation. Additionally, cD1 appears to be regulated by another proto-oncogene, c-myc. Studies have shown Myc can induce cD1 transcription and that ectopic Myc expression suppresses cD1 expression in cells that lack functional pRB (25, 31).
It has been difficult to identify individual cell cycle regulatory proteins to serve as reliable markers for prognosis in human lung cancer because of inconsistencies in studies in the literature. Our findings underscore the need to evaluate more than one protein that may affect the same pathway in order to predict prognosis. Because of these complex interactions, a molecular profile consisting of a battery of potential aberrant proteins for an individual lung cancer may be much more dependable in predicting long-term prognosis.
References
Landis SH, Murray T, Bolden S, Wingo PA . Cancer statistics, 1998. CA Cancer J Clin 1998; 48: 6–29.
Sherr CJ . Mammalian G 1 cyclins. Cell 1993; 73: 1059–1065.
Quelle DE, Ashmum RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, et al. Overexpression of mouse D type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev 1993; 7: 1559–1571.
Resnitzky D, Gossen M, Bujard H, Reed S . Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994; 14: 1669–1679.
Motokura T, Bloom T, Kim HG, Jupper H, Ruderman JV, Kronenberg HM, et al. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature 1991; 350: 512–515.
Pines J, Hunter T . Cyclins and cancer II: cyclin D1 and CDK inhibitors come of age. Cell 1994; 79: 573–582.
Jiang W, Zhang YJ, Kahn SM, Hollstein MC, Santella RM, Lu SH, et al. Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl Acad Sci U S A 1993; 90: 9026–9030.
Bellacosa A, Almadori G, Cavallo S, Cadoni G, Galli J, Ferrandina G, et al. Cyclin D1 amplification in human laryngeal squamous cell carcinomas: prognostic significance and clinical implications. Clin Cancer Res 1996; 2: 175–180.
Berenson JR, Yang J, Mickel RA . Frequent amplification of the bcl-1 locus in head and neck squamous cell carcinomas. Oncogene 1989; 4: 1111–1116.
Zhang YJ, Jiang W, Chen CJ, Lee CS, Kahn SM, Santella RM, et al. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun 1993; 196: 1010–1016.
Nishada N, Fukuda Y, Komeda T, Kita R, Sando T, Furukawa M, et al. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res 1994; 54: 3107–3110.
Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, Begg M, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 1996; 110: 669–674.
Weinstat-Saslow D, Merino MJ, Manrow RE, Lawrence JA, Bluth RF, Wittenbel KD, et al. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nature Med 1995; 1: 1257–1260.
Keyomarsi K, Pardee AB . Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A 1993; 90: 1112–1116.
Schuuring E, Verhoeven E, van Tinteren H, Peterse JL, Nunnink B, Thunnissen FB, et al. Amplification of genes within the chromosome 11q13 region is indicative of poor prognosis in patients with operable breast cancer. Cancer Res 1992; 52: 5229–5234.
Kwa HB, Michalides RJ, Dijkman JH, Mooi WJ . The prognostic value of NCAM, p53 and cyclin D1 in resected non-small cell lung cancer. Lung Cancer 1996; 14: 207–217.
Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, et al. A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer 1996; 73: 728–734.
Wang A, Yoshimi N, Suzui M, Yamauchi A, Tarao M, Mori H . Different expression patterns of cyclins A, D1 and E in human colorectal cancer. J Cancer Res Clin Oncol 1996; 122: 122–126.
Keyomarsi K, O'Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB . Cyclin E, a potential prognostic marker for breast cancer. Cancer Res 1994; 54: 380–385.
Bartek J, Lukas J, Strauss M . A common path to tumor growth: deregulation of Rb-dependent restriction point control [review]. Oncol Rep 1996; 3: 237–240.
Muller H, Lukas J, Schneider A, Warthoe P, Bartek J, Eilers M, et al. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Nat Acad Sci U S A 1994; 91: 2945–2949.
Geng Y, Eaton EN, Picon M, Roberts JM, Lundberg AS, Gifford A, et al. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene 1996; 12: 1173–1180.
Baldin V, Lukas J, Marcote MJ, Pagano M, Draette G . Cyclin D 1 is a nuclear protein required for cell cycle progression in G1. Genes Dev 1993; 7: 812–821.
Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M . Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol 1995; 15: 2612–2624.
Marhin WW, Hei YJ, Chen S, Jiang Z, Gallie BL, Phillips RA, et al. Loss of Rb and Myc activation co-operate to suppress cyclin D1 and contribute to transformation. Oncogene 1996; 12: 43–52.
Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J . Cyclin D 1 protein oscillates and is essential for cell cycle progression in human tumor cell lines. Oncogene 1994; 9: 707–718.
Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J . Cyclin D 1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol 1995; 15: 2600–2611.
Pagano M, Theodoras AM, Tam SW, Draetta G . Cyclin D 1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev 1994; 8: 1627–1639.
Bates S, Peters G . Cyclin D 1 as a cellular proto-oncogene. Sem Cancer Biol 1994; 6: 73–82.
Draetta G . Mammalian G 1 cyclins. Curr Opin Cell Biol 1994; 6: 842–846.
Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJZ . Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene 1994; 9: 3635–3645.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anton, R., Coffey, D., Gondo, M. et al. The Expression of Cyclins D1 and E in Predicting Short-Term Survival in Squamous Cell Carcinoma of the Lung. Mod Pathol 13, 1167–1172 (2000). https://doi.org/10.1038/modpathol.3880215
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880215
Keywords
This article is cited by
-
Combined expression of p53, cyclin D1 and epidermal growth factor receptor improves estimation of prognosis in curatively resected oral cancer
Modern Pathology (2005)
-
Prognostic value of cyclin D1 overexpression in correlation with pRb and p53 status in non-small cell lung cancer (NSCLC)
Journal of Cancer Research and Clinical Oncology (2005)
-
Cyclin D1, cyclin E, and p21 have no apparent prognostic value in anal carcinomas treated by radiotherapy with or without chemotherapy
British Journal of Cancer (2004)