Abstract
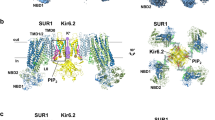
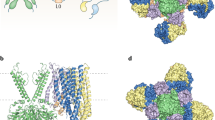
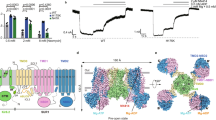
ATP-sensitive potassium channels (K-ATP channels) couple cell metabolism to electrical activity and are important in the physiology and pathophysiology of many tissues1. In pancreatic β-cells, K-ATP channels link changes in blood glucose concentration to insulin secretion2. They are also the target for clinically important drugs such as sulphonylureas, which stimulate secretion, and the K+ channel opener diazoxide, which inhibits insulin release3,4. Metabolic regulation of K-ATP channels is mediated by changes in intracellular ATP and Mg-ADP levels, which inhibit and activate the channel, respectively2. The β-cell K-ATP channel is a complex of two proteins5,6: an inward-rectifier K+ channel subunit, Kir6.2, and the sulphonylurea receptor, SUR1. We show here that the primary site at which ATP acts to mediate K-ATP channel inhibition is located on Kir6.2, and that SUR1 is required for sensitivity to sulphonylureas and diazoxide and for activation by Mg-ADP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Ashcroft, F. M. & Ashcroft, S.}. H. Properties and functions of ATP-sensitive K-channels. Cell. Signal 2, 197-214 (1990). 2. Ashcroft, F. M. & Rorsman, P. Electrophysiology of the pancreatic |3-cell. Prog. Biophys. Mol. Biol. 54, 87-143 (1989). 3. Ashcroft, F. M. & Ashcroft, S. J. H. The sulphonylurea receptor. Biochim. Biophys. Acta 1175,45-59 (1992). 4. Dunne, M. J., Harding, E., Jaggar, J. H., Ayton, B. J. & Squires, P. E. in Frontiers of Insulin Secretion and Pancreatic j3-Cell Research, (eds Flatt, P. & Lenzen, S.) 153-59 (Smith Gordon, UK, 1993). 5. Inagaki, N. etal. Reconstitution of IKATP: an inward rectifier subunit plus the sulphonylurea receptor. Science 270, 1166-1169 (1995). 6. Sakura, H., Ammala', C., Smith, P. A., Gribble, F. M. & Ashcroft, F. M. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic 0-cells, brain, heart and skeletal muscle. FEBS Lett. 377, 338-344 (1995). 7. Anguilar-Bryan, L. et al. Cloning of the /3-cell high-affinity sulphonylurea receptor: a regulator of insulin secretion. Science 268, 423-425 (1995). 8. Inagaki, N. et al. A family of sulfonylurea receptors determines the properties of ATP-sensitive K+ channels. Neuron 16, 1011-1017 (1996). 9. Ammala, C., Moorhouse, A. & Ashcroft, F. M. The sulphonylurea receptor confers diazoxide sensitivity on the inwardly-rectifying K-channel, Kir6.1. /. Physiol (Lond.) 494, 709-714 (1996). 10. Nichols, C. G. et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272, 1785-1787(1996). 11. Gribble, F. M., Tucker, S. J. & Ashcroft, F. M. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by MgADP and diazoxide. EMBO J. 16, 1145-1152 (1997). 12. Proks, P. & Ashcroft, F. M. Modification of K-ATP channels in pancreatic 0-cells by trypsin. Pfliigers Arch. 424, 63-72(1993). 13. Gribble, F. M., Ashfield, R., Ammala, C. & Ashcroft, F. M. Properties of cloned ATP-sensitive K-currents expressed in Xenopus oocytes. /. Physiol. (Lond.) 498, 87-98 (1997). 14. Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533-538 (1990). 15. Krapivinsky, G. etal. The G-protein-gated atrial K+ channel IKACH is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374, 135-141 (1995). 16. Sanguinetti, M. C. et al. Coassembly of KVLQT1 and MinK (IsK) proteins to form cardiac /KS potassium channel. Nature 384, 80-83 (1996). 17. Yamada, Y. M. et al. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP insensitive K+ channel./. Physiol. (Land.) 499, 715-720 (1997). 18. McNicholas, C. M., Yang, Y, Gebeisch, G. & Hebert, S. C. Molecular site for nucleotide binding on an ATP-sensitive renal K+ channel (ROMK2). Am. J. Physiol. 271, F275-F285 (1996). 19. Collins, A., German, M. S., Jan, Y. N., Jan, L. Y. & Zhao, B. A strongly inwardly rectifying K+ channel that is sensitive to ATP. /. Neurosci. 16, 1-9 (1996). 20. Higgins, C. F. ABC transporters: from microorganisms to man.Annu. Rev. CellBiol. 8,67-113 (1992). 21. Karschin, C., Ecke, C., Ahscroft, F. M. & Karschin, A. Overlapping tissue distribution of KATP channel-forming Kir6.2 subunit and the sulphonylurea receptor SUR1 in rodent brain. FEES Lett. 401,59-64 (1996). 22. Hilgemann, D. W., Nicoll, D. A. & Phillipson, K. D. Charge movement during Na+ translocation by native and cloned Na+/Ca2+ exchanger. Nature 352, 715-718 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tucker, S., Gribble, F., Zhao, C. et al. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387, 179–183 (1997). https://doi.org/10.1038/387179a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387179a0
This article is cited by
-
Structure of an open KATP channel reveals tandem PIP2 binding sites mediating the Kir6.2 and SUR1 regulatory interface
Nature Communications (2024)
-
Expression of truncated Kir6.2 promotes insertion of functionally inverted ATP-sensitive K+ channels
Scientific Reports (2021)
-
Association between KCNJ11 rs5219 variant and alcohol consumption on the effect of insulin secretion in a community-based Korean cohort: a 12-year follow-up study
Scientific Reports (2021)
-
New insights into KATP channel gene mutations and neonatal diabetes mellitus
Nature Reviews Endocrinology (2020)
-
Evaluating inositol phospholipid interactions with inward rectifier potassium channels and characterising their role in disease
Communications Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.