Abstract
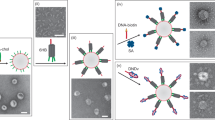
Vesicles of lipid bilayers have been investigated as drug-delivery vehicles for almost 20 years1-8. The vesicles’ interior space is separated from the surrounding solution because small molecules have only limited permeability through the bilayer. Single-walled (unilamellar) vesicles are made by a variety of non-equilibrium techniques, including mechanical disruption of lamellar phases by sonication or extrusion through filters, or chemical disruption by detergent dialysis or solvent removal5. These techniques do not, however, allow the encapsulation of a specific volume, nor can they be used to encapsulate other vesicles. Here we show that molecular-recognition processes mediated by lipophilic receptors and substrates (here the biotin–streptavidin complex)9 can be used to produce a multicompartmental aggregate of tethered vesicles encapsulated within a large bilayer vesicle. We can these encapsulated aggregates vesosomes. Encapsulation is achieved by unrolling bilayers from cochleate cylinders5,10-12 which are tethered to the aggregate by biotin–streptavidin coupling. These compartmentalized vesosomes could provide vehicles for multicomponent or multifunctional drug delivery2-4,6;in particular, the encapsulating membrane could significantly modify permeation properties, or could be used to enhance the biocompatibility of the system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bangham, A. D., Standish, M. M. & Watkins, J. C. Diffusion of univalent ions across lamellae of swollen phospholipids. J. Mol. Biol. 13, 238–252 (1965).
Lasic, D. D. Liposomes: from Physics to Applications (Elsevier, Amsterdam, 1993).
Gregoriadis, G. Liposomes as Drug Carriers—Recent Trends and Progress (Wiley, New York, 1988).
Fendler, J. Membrane Mimetic Chemistry (Wiley, New York, 1983).
Szoka, F. & Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 9, 467–508 (1980).
New, R. R. C. (ed.) Liposomes: a Practical Approach (Oxford Univ. Press, 1990).
Spector, M. S., Zasadzinski, J. A. & Sankaram, M. B. Topology of multivesicular liposomes, a model biliquid foam. Langmuir 12, 4704–4708 (1996).
Allen, T. M., Hansen, C. B. & Lopes de Menezes, D. E. Pharmacokinetics of long-circulating liposomes. Adv. Drug Delivery Rev. 16, 267–284 (1995).
Chiruvolu, S., Walker, S., Leckband, D., Israelachvili, J. & Zasadzinski, J. Higher order self-assembly of vesicles by ligand-receptor interactions. Science 264, 1753–1756 (1994).
Papahadjopoulos, D., Vail, W. J., Jacobson, K. & Poste, G. Cochleate lipid cylinders formation by fusion of unilamellar lipid vesicles. Biochim. Biophys. Acta 394, 483–491 (1975).
Papahadjopoulos, D., Vail, W. J., Pangborn, W. A. & Poste, G. Studies on membrane fusion II: induction of fusion in pure phospholipid membranes by calcium ions and other divalent ions. Biochim. Biophys. Acta 448, 265–283 (1976).
Coossen, J. R. & Rand, R. P. Structural effects of neutral lipids on divalent cation-induced interactions of phosphatidylserine-containing bilayers. Biophys. J. 68, 1009–1018 (1995).
Papahadjopoulos, D. et al. Studies on membrane fusion III: the role of calcium-induced phase changes. Biochim. Biophys. Acta 465, 579–598 (1977).
Allen, T. M. & Chonn, A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 223, 42–46 (1987).
Uster, P. S. et al. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 386, 243–246 (1996).
Zalipsky, S., Hansen, C. B., Lopes de Menezes, D. E. & Allen, T. M. Long-circulating, polyethylene glycol grafted immunopoliposimes. J. Controlled Release 39, 153–161 (1996).
Wong, J. Y., Kuhl, T. L., Israelachvili, J. N., Mullah, N. & Zalipsky, S. Direct measurement of a tethered ligand-receptor interaction potential. Science 275, 820–822 (1997).
Walker, S. A. thesis, Univ. California, Santa Barbara, (1996).
Kennedy, M. T. thesis, Univ. California, Santa Barbara (1998).
Tardi, P. G., Boman, N. L. & Cullis, P. R. Liposomal doxorubicin. J. Drug Targeting 4, 129–140 (1996).
Papahadjopolous, D. et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antiturmour therapeutic efficacy. Proc. Natl Acad. Sci. USA 88, 11460–11464 (1991).
Chiruvolu, S., Naranjo, E. & Zasadzinski, J. A. in Structure and Flow in Surfactant Solutions Ch. 5 (eds Herb, C. A. & Prud’homme, R. K.) (Am. Chem. Soc., Washington DC, 1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walker, S., Kennedy, M. & Zasadzinski, J. Encapsulation of bilayer vesicles by self-assembly. Nature 387, 61–64 (1997). https://doi.org/10.1038/387061a0
Issue Date:
DOI: https://doi.org/10.1038/387061a0
This article is cited by
-
Relevance of multilamellar and multicompartmental vesicles in biological fluids: understanding the significance of proportional variations and disease correlation
Biomarker Research (2023)
-
Hydrogels as functional components in artificial cell systems
Nature Reviews Chemistry (2022)
-
Membrane functionalization in artificial cell engineering
SN Applied Sciences (2020)
-
Folate Conjugated Double Liposomes Bearing Prednisolone and Methotrexate for Targeting Rheumatoid Arthritis
Pharmaceutical Research (2019)
-
Lectin-mediated protocell crosslinking to mimic cell-cell junctions and adhesion
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.