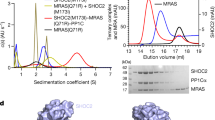
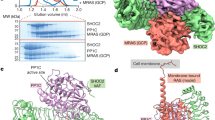
Abstract
RAS-RELATED GTP-binding proteins function as molecular switches which cycle between GTP-bound 'on'- and GDP-bound 'off'-states1. GTP hydrolysis is the common timing mechanism that mediates the return from the 'on' to the 'off'-state. It is usually slow but can be accelerated by orders of magnitude upon inter-action with GTPase-activating proteins (GAPs). In the case of Ras, a major regulator of cellular growth, point mutations are found in approximately 30% of human tumours which render the protein unable to hydrolyse GTP, even in the presence of Ras-GAPs. The first structure determination of a GTPase-activating protein reveals the catalytically active fragment of the Ras-specific p120GAP (ref. 2), GAP-334, as an elongated, exclusively helical protein which appears to represent a novel protein fold. The molecule consists of two domains, one of which contains all the residues conserved among different GAPs for Ras. From the location of conserved residues around a shallow groove in the central domain we can identify the site of interaction with Ras·GTP. This leads to a model for the interaction between Ras and GAP that satisfies numerous biochemical and genetic data on this important regulatory process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bourne, H. R., Sanders, D. A. & McCormick, F. Nature 349, 117–127 (1991).
Trahey, M. & McCormick, F. Science 238, 542–545 (1987).
Ahmadian, M. R., Wiesmüller, L., Lautwein, A., Bischoff, F. R. & Wittinghofer, A. J. Biol. Chem. 271, 16409–16415 (1996).
Vogel, U. et al. Nature 335, 90–93 (1988).
Trahey, M. et al. Science 242, 1697–1700 (1988).
Henkemeyer, M. et al. Nature 377, 695–701 (1995).
Xu, G. et al. Cell 62, 599–608 (1990).
Li, Y. et al. Cell 69, 275–281 (1992).
Scheffzek, K., Lautwein, A., Scherer, A., Franken, S. & Wittinghofer, A. Proteins Struct. Func. Genet. (in the press).
Coleman, D. E. et al. Science 265, 1405–1412 (1994).
Sondek, J., Lambright, D. G., Noel, J. P., Hamm, H. E. & Sigler, P. B. Nature 372, 276–279 (1994).
Markby, D. W., Onrust, R. & Bourne, H. R. Science 262, 1895–1901 (1993).
Mittal, R., Ahmadian, M. R., Goody, R. S. & Wittinghofer, A. Science 273, 115–117 (1996).
Miao, W., Eichelberger, L., Baker, L. & Marshall, M. S. J. Biol. Chem. 271, 15322–15329 (1996).
Gutmann, D. H. et al. Oncogene 8, 761–769 (1993).
Nishi, T. et al. Oncogene 6, 1555–1559 (1991).
Rey, I., Taylor-Haris, P., van Erp, H. & Hall, A. Oncogene 9, 685–692 (1994).
Hettich, L. & Marshall, M. Cancer Res. 54, 5438–5444 (1994).
Brownbridge, G. G., Lowe, P. N., Moore, K. J. M., Skinners, R. H. & Webb, M. R. J. Biol. Chem. 268, 10914–10919 (1993).
Morcos, P., Thapar, N., Tusneem, N., Stacey, D. & Tamanoi, F. Mol. Cell. Biol. 16, 2496–2503 (1996).
Poullet, P., Lin, B., Esson, K. & Tamanoi, F. Mol. Cell. Biol. 14, 815–821 (1994).
Wiesmüller, L. & Wittinghofer, A. J. Biol. Chem. 267, 10207–10210 (1992).
Parrini, M. C., Bernardi, A. & Parmeggiani, A. EMBO J. 15, 1107–1111 (1996).
Yoder-Hill, J., Golubic, M. & Stacey, D. W. J. Biol. Chem. 270, 27615–27621 (1995).
Rubinfeld, B. et al. Int. J. Pept. Protein Res. 38, 47–53 (1991).
Pai, E. F. et al. EMBO J. 9, 2351–2359 (1990).
Mori, S. et al. J. Biol. Chem. 270, 28834–28838 (1995).
Nassar, N. et al. Nature 375, 554–560 (1995).
Moore, K. J. M., Webb, M. R. & Eccleston, J. F. Biochemistry 32, 7451–7459 (1993).
Rensland, H., Lautwein, A., Wittinghofer, A. & Goody, R. S. Biochemistry 30, 11181–11185 (1991).
Kabsch, W. J. Appl. Crystallogr. 26, 795–800 (1993).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjelgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, T. A. XPLOR version 3.1 (Yale University, 1992).
Kabsch, W. & Sander, C. Biopolymers 22, 2577–2637 (1983).
Kraulis, P. J. Appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scheffzek, K., Lautwein, A., Kabsch, W. et al. Crystal structure of the GTPase-activating domain of human p120GAP and implications for the interaction with Ras. Nature 384, 591–596 (1996). https://doi.org/10.1038/384591a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/384591a0
This article is cited by
-
A novel protein RASON encoded by a lncRNA controls oncogenic RAS signaling in KRAS mutant cancers
Cell Research (2022)
-
Prognostic values, ceRNA network, and immune regulation function of SDPR in KRAS-mutant lung cancer
Cancer Cell International (2021)
-
Identification of an individual with a SYNGAP1 pathogenic mutation in India
Molecular Biology Reports (2020)
-
Computational insights of K1444N substitution in GAP-related domain of NF1 gene associated with neurofibromatosis type 1 disease: a molecular modeling and dynamics approach
Metabolic Brain Disease (2018)
-
The C2 domain of SynGAP is essential for stimulation of the Rap GTPase reaction
EMBO reports (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.