Abstract
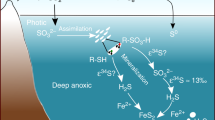
THE sulphur cycle has evolved over the course of the Earth's history1,2. The early Earth's surface environment was reducing, containing little atmospheric oxygen3, and with seawater sulphate concentrations estimated at less than a few per cent of those found today. The accumulation of sulphate in the ocean to much higher concentrations was probably coincident with the initial accumulation of oxygen in the atmosphere and the consequent oxidative weathering of sulphide minerals on land4,5. Past changes in sulphate concentrations in ancient oceans have previously been assessed by comparing the systematics of sulphur isotope fractionation by sulphate-reducing bacteria6–9 with the isotopic composition of sedimentary sulphides1,2,5,10,11. But such interpretations have proven equivocal: the generally small 34S depletions in Archaean sulphides (deposited ∼2.5–3.8 billion years ago) have been separately argued to result both from rapid sulphate reduction in a sulphate-rich ocean5,12, and from sulphide formation in a sulphate-poor ocean1,2,11. Here we report large 34S depletions of 20–25%, observed during rapid sulphate reduction by sulphate-reducing bacteria in modern photosynthetic cyano-bacterial mats from Solar Lake, Sinai. We conclude that high sulphate concentrations give rise to highly 34S-depleted sulphides, and thus that appreciable concentrations of seawater sulphate did not accumulate until the initial accumulation of oxygen into the atmosphere in post-Archaean times.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schidlowski, M., Hayes, J. M. & Kaplan, I. R. in Earth's Earliest Biosphere: Its Origin and Evolution (ed. Schopf, J. W.) 149–186 (Princeton Univ. Press, Princeton, New Jersey, 1983).
Cameron, E. M. Nature 296, 145–148 (1982).
Kasting, J. F. Science 259, 920–926 (1993).
Walker, J. C. G. & Brimblecombe, P. Precambr. Res. 28, 205–222 (1985).
Ohmoto, H., Kakegawa, T. & Lowe, D. R. Science 262, 555–557 (1993).
Kaplan, I. R. & Rittenberg, S. C. J. gen. Microbiol. 34, 195–212 (1964).
Kemp, A. L. W. & Thode, H. G. Geochim. cosmochim. Acta 32, 71–91 (1968).
Chambers, L. A., Trudinger, P. A., Smith, J. W. & Burns, M. S. Can. J. Microbiol. 21, 1602–1607 (1975).
Goldhaber, M. B. & Kaplan, I. R. Soil Sci. 119, 42–55 (1975).
Goodwin, A. M., Monster, J. & Thode, H. G. Econ. Geol. 71, 870–891 (1976).
Hattori, K., Krouse, H. R. & Campbell, F. A. Science 221, 549–551 (1983).
Ohmoto, H. & Felder, R. P. Nature 328, 244–246 (1987).
Harrison, A. G. & Thode, H. G. Trans. Faraday Soc. 54, 84–92 (1958).
Canfield, D. E. & Teske, A. Nature 382, 127–132 (1996).
Harrison, A. G. & Thode, H. G. Trans. Faraday Soc. 53, 1648–1651 (1957).
Schidlowski, M. Origins of Life 9, 299–311 (1979).
Hattori, K., Campbell, F. A. & Krouse, H. R. Nature 302, 323–326 (1983).
Lambert, I. B. & Donnelly, T. H. in Stable Isotopes and Fluid Processes in Mineralization (eds Herbert, H. K. & Ho, S. E.) 260–268 (Geology Department and Unviersity Extension, Perth, Australia, 1990).
Monster, J. et al. Geochim. cosmochim. Acta 43, 405–413 (1979).
Donnelly, T. H. et al. J. geol. Soc. Australia 24, 409–420 (1977).
Hayes, J. M., Lambert, I. B. & Strauss, H. in The Proterozoic Biosphere. A Multidisciplinary Study (eds Schopf, J. W. & Klein, C.) 129–132 (Cambridge Univ. Press, Cambridge, 1992).
Canfield, D. E. & Thamdrup, B. Science 266, 1973–1975 (1994).
Des Marais, D. J. Trends Ecol. Evol. 5, 140–143 (1990).
Jørgensen, B. B. FEMS Microbiol. Ecol. 13, 303–312 (1994).
Canfield, D. E. & Des Marais, D. J. Geochim. cosmochim. Acta 57, 3971–3984 (1993).
Harrison, A. W. et al. in Coastal Upwelling (ed. Richards, F. A.) 303–311 (American Geophysical Union, Washington DC, 1981).
Revsbech, N. P., Jørgensen, B. B. & Blackburn, T. H. Limnol. Oceanogr. 28, 1062–1074 (1983).
Jørgensen, B. B. & Cohen, Y. Limnol. Oceanogr. 22, 657–666 (1977).
Des Marais, D. J. et al. Nature 359, 605–609 (1992).
Holland, H. D. in Early Life on Earth (ed. Bengston, S.) 237–244 (Columbia University Press, New York, 1994).
Canfield, D. E. et al. Chem. Geol. 54, 149–155 (1986).
Cameron, E. M. & Hattori, K. Chem. Geol. 65, 341–358 (1987).
Cline, J. D. Limnol. Oceanogr. 14, 454–458 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Habicht, K., Canfield, D. Sulphur isotope fractionation in modern microbial mats and the evolution of the sulphur cycle. Nature 382, 342–343 (1996). https://doi.org/10.1038/382342a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/382342a0
This article is cited by
-
Micron-scale mapping of sulfur cycling across the oxycline of a cyanobacterial mat: a paired nanoSIMS and CARD-FISH approach
The ISME Journal (2008)
-
Authigenic sulfide minerals and their sulfur isotopes in sediments of the northern continental slope of the South China Sea and their implications for methane flux and gas hydrate formation
Chinese Science Bulletin (2007)
-
Identifying microorganisms responsible for ecologically significant biogeochemical processes
Nature Reviews Microbiology (2005)
-
The habitat and nature of early life
Nature (2001)
-
A new model for Proterozoic ocean chemistry
Nature (1998)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.