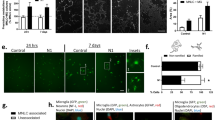
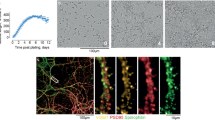
Abstract
THE prion protein PrPc is a glycoprotein of unknown function1 normally found in neurons2 and glia3. It is involved in diseases such as bovine spongiform encephalopathy (BSE), scrapie and Creutzfeldt–Jakob disease4. PrPSc, an altered isoform of PrPc that is associated with disease, shows greater protease resistance and is part of the infectious agent, the prion5,6. Prion diseases are characterized by neuronal degeneration, gliosis and accumulation of PrPSc (ref. 7). Mice devoid of PrPc are resistant to scrapie8. A fragment of human PrP consisting of amino acids 106–126 that forms fibrils in vitro is toxic to cultured neurons9–11. Here we show that this toxic effect requires the presence of microglia which respond to PrP106–126 by increasing their oxygen radical production. The combined direct and microglia-mediated effects of PrP106–126 are toxic to normal neurons but are insufficient to destroy neurons from mice not expressing PrPc.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Herms, J. W., Kretzschmar, H. A., Titz, S. & Keller, B. U. Eur. J. Neurosci. 7, 2508–2512 (1995).
Kretzschmar, H. A., Prusiner, S. B., Stowring, L. E. & DeArmond, S. J. Am. J. Path. 122, 1–5 (1986).
Moser, M., Colello, R. J., Pott, U. & Oesch, B. Neuron 14, 509–517 (1995).
Prusiner, S. B. Science 252, 1515–1522 (1991).
Bolton, D. C., McKinley, M. P. & Prusiner, S. B. Science 218, 1309–1311 (1982).
Caughey, B. & Raymond, G. J. J. biol. Chem. 266, 18217–18223 (1991).
DeArmond, S. J. et al. Neurology 37, 1271–1280 (1987).
Büeler, H. et al. Cell 73, 1339–1347 (1993).
Forloni, G. et al. Nature 362, 543–546 (1993).
Brown, D. R., Herms, J. & Kretzschmar, H. A. Neuroreport 5, 2057–2060 (1994).
Selvaggini, C. et al. Biochem. biophys. Res. Commun. 194, 1380–1386 (1993).
Büeler, H. et al. Nature 356, 577–582 (1992).
Giulian, D. & Baker, T. J. J. Neurosci. 6, 2163–2178 (1986).
Giulian, D. et al. J. Neurosci. 8, 709–714 (1988).
Betz Corradin, S. et al. Glia 7, 255–262 (1993).
Beckman, J. S. et al. Proc. natn. Acad. Sci. U.S.A. 87, 1620–1624 (1990).
Müller, W. E. G. et al. Eur. J. Pharmac. 246, 261–267 (1993).
Giese, A., Groschup, M., Hess, B. & Kretzschmar, H. A. Brain Path. 5, 213–221 (1995).
Williams, A. E., Lawson, L. J., Perry, V. H. & Fraser, H. Neuropath, appl. Neurobiol. 20, 47–55 (1994).
Meda, L. et al. Nature 374, 647–650 (1995).
Frackowiak, J. et al. Acta neuropath. 84, 225–233 (1992).
Elstner, E. F. & Heupel, A. Ann. Biochem. 70, 616–620 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, D., Schmidt, B. & Kretzschmar, H. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380, 345–347 (1996). https://doi.org/10.1038/380345a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/380345a0
This article is cited by
-
Lack of Cathepsin D in the central nervous system results in microglia and astrocyte activation and the accumulation of proteinopathy-related proteins
Scientific Reports (2022)
-
A Dielectric Barrier Discharge Plasma Degrades Proteins to Peptides by Cleaving the Peptide Bond
Plasma Chemistry and Plasma Processing (2020)
-
Regulation of the aggregation behavior of human islet amyloid polypeptide fragment by titanocene complexes
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Celecoxib Inhibits Prion Protein 90-231-Mediated Pro-inflammatory Responses in Microglial Cells
Molecular Neurobiology (2016)
-
Toll-Like Receptor 2 Deficiency Shifts PrP106-126-Induced Microglial Activation from a Neurotoxic to a Neuroprotective Phenotype
Journal of Molecular Neuroscience (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.