Abstract
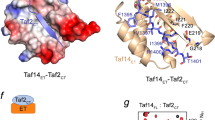
A complex of two TFIID TATA box-binding protein-associated factors (TAFIIs) is described at 2.0 Å resolution. The amino-terminal portions of dTAFII42 and dTAFII62 from Drosophila adopt the canonical histone fold, consisting of two short α-helices flanking a long central α-helix. Like histones H3 and H4, dTAFII42 and dTAFII62 form an intimate heterodimer by extensive hydrophobic contacts between the paired molecules. In solution and in the crystalline state, the dTAFII42/dTAFII62 complex exists as a heterotetramer, resembling the (H3/H4)2 heterotetrameric core of the histone octamer, suggesting that TFIID contains a histone octamer-like substructure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Roeder, R. G. Trends biochem. Sci 16, 402–408 (1991).
Hori, R. & Carey, M. Curr. Opin. Genet. Dev. 4, 236–244 (1994).
Zawel, L. & Reinberg, D. Prog. Nucleic Acid Res. molec. Biol. 44, 67–108 (1993).
Zawel, L., Kumar, K. & Reinberg, D. Genes Dev. 9, 1479–1490 (1995).
Koleske, A. & Young, R. Trends biochem. Sci. 20, 113–116 (1995).
Matsui, T., Segall, J., Weil, P. & Roeder, R. G. J. biol. Chem. 255, 11992–11996 (1980).
Sawadogo, M. & Roeder, R. G. Cell 43, 165–175 (1985).
Kim, J. L. & Burley, S. K. Nature Struct. Biol. 1, 638–653 (1994).
Burley, S. K. & Roeder, R. G. A. Rev. Biochem. 65 (in the press).
Owen-Hughes, T. & Workman, J. L. Crit. Rev. Euk. Gene Express. 4, 403–441 (1994).
Nikolov, D. B. & Burley, S. K. Nature Struct. Biol. 1, 621–637 (1994).
Reese, J., Apone, L., Walker, S., Griffin, L. & Green, M. Nature 371, 523–527 (1994).
Poon, D. et al. Proc. natn. Acad. Sci. U.S.A. 92, 8224–8228 (1995).
Chen, J., Attardi, L., Verrijzer, D., Yokomori, K. & Tijan, R. Cell 79, 93–105 (1994).
Sauer, F., Hansen, S. & Tijan, R. Science 270, 1783–1788 (1995).
Kokubo, T. et al. Nature 367, 484–487 (1994).
Hisatake, K. et al. Proc. natn. Acad. Sci. U.S.A. 92, 8195–8199 (1995).
Hoffmann, A. et al. Nature 380, 356–359 (1996).
Baxevanis, A., Arents, G., Moudrianakis, E. & Landsman, D. Nucleic Acids Res. 23, 2685–2691 (1995).
Goodrich, J., Hoey, T., Thut, C., Admon, A. & Tjian, R. Cell 75, 519–30 (1993).
Weinzierl, R., Ruppert, S., Dynlacht, B., Tanese, N. & Tjian, R. EMBO J. 12, 5303–5309 (1993).
Mengus, G. et al. EMBO J. 14, 1520–1531 (1995).
Yokomori, K., Chen, J.-L., Admon, A., Zhou, S. & Tjian, R. Genes Dev. 7, 2587–2597 (1993).
Clark, K. L., Halay, E. D., Lai, E. & Burley, S. K. Nature 364, 412–420 (1993).
Ramakrishnan, V., Finch, J., Graziano, V. & Sweet, R. Nature 362, 219–223 (1993).
McPherson, C., Shin, E.-Y., Friedman, D. & Zaret, K. Cell 75, 387–398 (1993).
D'Arcy, A. Acta crystallogr. D50, 469–411 (1994).
Ferré-D'Amaré, A. R. & Burley, S. K. Structure 2, 357–359, 567 (1994).
Cohen, S. L., Ferre-D'Amare, A. R., Burley, S. K. & Chait, B. T. Protein Sci. 4, 1088–1099 (1995).
Lattman, E., Burlingame, R., Hatch, C. & Moudrianakis, E. N. Science 216, 1016–1018 (1982).
Brünger, A. T. X-PLOR, Version 3.1 manual (Yale University, New Haven, CT, 1992).
Laskowski, R. J., Macarthur, M. W., Moss, D. S. & Thornton, J. M. J. appl. Crystallogr. 26, 283–290 (1993).
Arents, G. & Moudrianakis, E. Proc. natn. Acad. Sci. U.S.A. 92, 11170–11174 (1995).
Arents, G., Burlingame, R. W., Wang, B.-C., Love, W. E. & Moudrianakis, E. N. Proc. natn. Acad. Sci. U.S.A. 88, 10148–10152 (1991).
Karantza, V., Friere, E. & Moudrianakis, E. N. Biochemistry 35 (in the press).
Janin, J. Proteins 21, 30–39 (1995).
Nikolov, D. B. et al. Nature 377, 119–128 (1995).
Klemm, R., Goodrich, J., Zhou, S. & Tjian, R. Proc. natn. Acad. Sci. U.S.A. 92, 92, 5788–5792 (1995).
Thut, C., Chen, J. L., Klemm, R. & Tjian, R. Science 267, 100–104 (1995).
Lu, H. & Levine, A. Proc. natn. Acad. Sci. U.S.A. 92, 5154–5158 (1995).
Horikoshi, M., Carey, M., Kadidani, H. & Roeder, R. G. Cell 54, 665–669 (1988).
Horikoshi, M., Hai, T., Lin, Y.-S., Green, M. R. & Roeder, R. G. Cell 54, 1033–1042 (1988).
Lieberman, P. & Berk, A. Genes Dev. 8, 995–1006 (1994).
Chi, T., Lieberman, P., Ellwood, K. & Carey, M. Nature 377, 254–257 (1995).
Tjian, R. & Maniatis, T. Cell 77, 5–8 (1994).
Klug, A., Rhodes, D., Smith, J., Finch, J. & Thomas, J. Nature 287, 509–516 (1980).
Richmond, T., Finch, J., Rushton, B., Rhodes, D. & Klug, A. Nature 311, 532–537 (1984).
Arents, G. & Moudrianakis, E. N. Proc. natn. Acad. Sci. U.S.A. 90, 10489–10493 (1993).
Pruss, D., Hayes, J. & Wolffe, A. BioEssays 17, 161–170 (1995).
Hendrickson, W. A. Science 254, 51–58 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xie, X., Kokubo, T., Cohen, S. et al. Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature 380, 316–322 (1996). https://doi.org/10.1038/380316a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/380316a0
This article is cited by
-
Chaperonin CCT checkpoint function in basal transcription factor TFIID assembly
Nature Structural & Molecular Biology (2018)
-
Structure of promoter-bound TFIID and model of human pre-initiation complex assembly
Nature (2016)
-
Structural basis of transcription initiation by RNA polymerase II
Nature Reviews Molecular Cell Biology (2015)
-
Cytoplasmic TAF2–TAF8–TAF10 complex provides evidence for nuclear holo–TFIID assembly from preformed submodules
Nature Communications (2015)
-
The architecture of human general transcription factor TFIID core complex
Nature (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.