Abstract
Giant-cell tumor of bone is considered a benign, locally aggressive and rarely metastasizing neoplasm of bone. Specific microscopic or radiographic findings that reliably predict behavior have remained elusive. However, recent evidence suggests that activity of the telomerase enzyme complex correlates with recurrence in giant-cell tumor, although the subset of cells with telomerase activity in these heterogeneous tumors has not been defined. In the present study, we investigated whether immunostaining for human telomerase reverse transcriptase, a component of the telomerase complex, correlates with outcome in giant-cell tumor and the distribution of telomerase reverse transcriptase staining in these tumors. We analyzed 58 cases of giant-cell tumor for the presence and pattern of telomerase reverse transcriptase immunostaining, presence of soft tissue involvement and the type of initial surgery, and correlated these findings with recurrence-free survival and metastasis-free survival. Specific staining with telomerase reverse transcriptase was present in 20 out of 58 tumors (35%) in the nuclei of mononuclear cells and, occasionally, osteoclast-like giant cells. Furthermore, positive telomerase reverse transcriptase immunohistochemistry correlated with recurrence-free survival (P=0.02), whereas the presence of soft tissue extension (P=0.3) and the type of initial surgery (P=0.2) did not. Only soft-tissue extension significantly correlated with metastasis-free survival (P=0.003). Therefore, telomerase reverse transcriptase expression may predict recurrence in giant-cell tumor insofar as positive immunostaining correlates with shorter recurrence-free survival and may be a useful prognostic marker to stratify patients to more aggressive treatment protocols.
Similar content being viewed by others
Main
Giant-cell tumor of bone is a distinct clinical-radio-pathologic entity with the biologic potential for locally aggressive growth and rare metastases. The risk of local recurrence and distant metastasis for giant-cell tumor has been estimated to be 25 and 2%, respectively, but the clinical behavior of any given case of giant-cell tumor is difficult to predict.1, 2, 3, 4 The occasionally delayed recurrence of these tumors can also confound studies of clinical outcome.5 Some reports suggest that recurrent giant-cell tumor may predict a higher risk of malignant transformation.2, 6 Controversies exist regarding treatment protocols.7 Thus, parameters that predict recurrence potential at initial diagnosis may be clinically useful to guide management.
Prognostic markers of giant-cell tumor have remained elusive. Unfortunately, the usual light-microscopic features associated with aggressive clinical behavior in most neoplasms, including necrosis, mitotic activity and even vascular invasion, do not predict outcome in giant-cell tumor.1, 8 The discrepancy between histopathology and clinical course may stem from the heterogeneous population of cells that constitute giant-cell tumor. More specifically, culture experiments suggest that the neoplastic cells of giant-cell tumor represent only a small subset of a mixed population of cells that include monocyte–macrophages, osteoclast-like giant cells and others.9, 10, 11, 12 The neoplastic cells have features of primitive osteoblast precursors that cytogenetically demonstrate telomeric association.
Conflicting data exist on whether radiologic grade (Campanacci grade) correlates with recurrence.3 In some reports, Campanacci grade III tumors demonstrated more aggressive behavior,13, 14 although statistical correlation has not been observed in other studies.15, 16, 17 Campanacci grade III is defined as having indistinct borders on plain radiographs,3 which generally, but not exclusively, predicts soft-tissue involvement histologically. Some authors have suggested that radiologic grade simply predicts the likelihood of adequate treatment, and it is the latter that influences clinical behavior.8 Giant-cell tumor is typically treated with intralesional curettage, burring and packing with cement or bone graft. More aggressive treatment protocols involve resection so as to achieve wider margins than are customarily accomplished with intralesional curettage procedures.
Activation of the enzyme telomerase, a strategy that overcomes senescence barriers by stabilizing telomere length, is required for cellular immortalization and transformation, and may contribute to cell immortality.18, 19 Telomerase activity has been observed in up to 90% of human malignancies.20 In giant-cell tumor, telomerase activity has been studied using cell culture,21 and from tumor cell lysates, using the telomere repeat amplification protocol (TRAP) assay. In a recent study, Matsuo et al17 used TRAP assay to show that the level of telomerase activity correlates with local recurrence in giant-cell tumor. Telomerase activity depends on transcriptional induction of human telomerase reverse transcriptase (hTERT).22 Consequently, some investigators have used immunohistochemistry for hTERT protein as a substitute for the TRAP assay. In some mesenchymal tumors, for example, hTERT expression correlates with aggressive behavior.23, 24, 25, 26, 27, 28, 29 Taken together, the above studies suggest that immunohistochemistry for hTERT could be used to predict aggressive behavior in giant-cell tumor of bone. Immunohistochemistry also allows direct visualization of the specific cell types with telomerase activity, a characteristic, which may be informative in heterogeneous tumors such as giant-cell tumor. Whereas most somatic cells do not express telomerase, its activity can be detected in differentiating and activated lymphocytes.30 Thus, the contribution of lymphocytes to TRAP results cannot be resolved from homogenized lysates.31
The purpose of this study was to examine the pattern and prognostic significance of telomerase expression in a group of well-characterized giant-cell tumor of bone treated at a single institution. We employed immunohistochemical staining for hTERT expression in paraffin-embedded sections of giant-cell tumor, and correlated the results with recurrence and metastasis. We also determined whether the correlation with outcome was independent of soft-tissue extension or the initial type of surgery.
Materials and methods
Selection of Cases
Fifty-eight consecutive patients with giant-cell tumor were identified from the Department of Pathology files of the University of California, San Francisco. The diagnosis was based on light-microscopic, radiographic and clinical features. To consistently define the criteria for case selection, all patients were skeletally mature as judged by plain radiographs, and all long-bone tumors involved the epiphysis. We planned to exclude patients with other documented neoplasms that may produce pulmonary metastasis thereby confounding the outcome analysis, but no patients met this criterion. Histologically, all primary and recurrent tumors lacked high-grade nuclear features or atypical mitotic forms to suggest malignant giant-cell tumor.6, 32, 33
Immunohistochemistry
Immunohistochemical analysis was performed using standard techniques34 on non-decalcified, paraffin-embedded sections of giant-cell tumor. Briefly, 4-μm paraffin-embedded sections were deparaffinized, heated in citrate buffer, blocked for endogenous peroxidase, avidin and biotin, incubated with a monoclonal antibody to telomerase reverse transcriptase at 1:25 dilution (ALX-804-504; Alexis, San Diego, CA, USA), washed and developed using the ABC kit (Vector Laboratories, Burlingame, CA, USA). The primary antibody dilution was based on optimization using normal human tonsil tissue. In many cases, staining was patchy, as has been previously reported for hTERT in other tumors.24 Thus, we converted staining to a dichotomous variable to maximize the ability to predict clinical outcome: cases with ≥10% of cells showing nuclear and/or nucleolar staining were scored as positive.
Clinicopathological Factors
All available radiographic images, histological slides and reports were reviewed. Soft-tissue extension was recorded as either present or absent, to correlate with recurrence-free survival and/or metastasis-free survival. We incorporated histological evidence of a soft-tissue component, since radiographic grading does not universally correlate with histological findings.1 Further, histological confirmation of soft-tissue extension avoids some of the ambiguity of whether a thin shell of bone radiographically (Campanacci grade II)3 represents residual, lamellar, cortical bone, or a thin rim of reactive woven bone. The type of initial surgery, curettage or en bloc resection was determined by review of the clinical records. Identification of recurrence or metastasis was based on review of the clinical record, radiographic findings and pathology of metastectomy specimens, when available.
Statistical Analysis
Recurrence-free survival and metastasis-free survival were defined as the time between diagnosis and the first local recurrence or the first metastasis, respectively, or the last follow-up visit. All patients were alive at the end of the study; thus, no patients were censored for death. Univariate analysis for metastasis-free survival and recurrence-free survival rates was conducted using the Kaplan–Meier method,7 and the log-rank test was used for comparisons with alpha set to P=0.05. Only one significant factor was identified for each outcome using univariate analysis. Thus, multivariate analysis was not evaluated.
Results
Clinical and Pathological Features
Clinical and pathologic features are summarized in Table 1. The patients consisted of 33 men and 25 women who ranged in age from 17 to 66 years (average 35 years). In most cases, patients presented with relatively well-circumscribed, expansile, lytic lesions extending to the epiphyses of long bones. The most common site was the distal femur (n=21, 36%). Two patients had multifocal involvement. Histologically, all tumors showed areas diagnostic of giant-cell tumor as defined by the WHO Classification of tumors of bone and soft tissue,1 including sheets of uniform oval mononuclear cells with relatively evenly distributed osteoclast-like giant cells sharing similar nuclear morphology, and generally without matrix production (Figure 1a). No overt evidence of malignancy, such as high-grade cytologic atypia or atypical mitotic figures, was identified in any case. Soft-tissue extension was present in 28 cases (48%), which was confirmed by a combination of radiographic and histological findings, as outlined in Materials and methods (Figure 1b and c).
Treatment for the majority of tumors (50 tumors, 86%) was curettage followed by packing with bone graft or cement. Eight tumors (14%) were treated with en bloc resection as the initial surgery. A single patient (case 20) with extensive disease at the time of initial diagnosis that precluded reconstruction options, underwent primary amputation. Adjuvant therapy with interferon-alpha was documented for patients 20 and 29, but only after evidence of metastasis. None of the patients received cytotoxic chemotherapy or radiotherapy. Average follow-up duration was 25 months (range 1–77 months).
Nine patients (16%) had recurrence, with an average recurrence-free survival of 15 months (range 11–39 months); three of these patients (5%) had multiple recurrences. Nine patients (16%) had metastases, all pulmonary, with average metastasis-free survival of 9 months (range 0–36 months). Patients 26, 30 and 57 had metastases at the time of diagnosis of the primary tumor (ie, 0-month metastasis-free survival). Patients 29, 30 and 42 underwent metastectomy. All of the patients, regardless of metastatic or recurrence status, were alive at the end of the study.
Immunohistochemistry
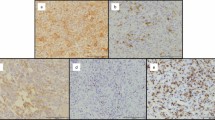
Strong nuclear staining for hTERT was identified in 20 tumors (35%) in the mononuclear cells and, in some cases, a subset of the nuclei of osteoclast-like giant cells. The pattern of staining within a given tumor was patchy, as has been previously reported,24 but positive cases generally stained >50% of the cells (Figure 2a and b). Some of the tumors scored as negative (<10% of cells staining) demonstrated an occasional small lymphocyte with nuclear staining, which served as an internal positive control for the immunohistochemistry. Apart from an occasional lymphocyte, areas resembling benign fibrous histiocytoma, with storiform growth of spindle cells and foamy histiocytes,35 were uniformly negative for hTERT (Figure 2c).
Representative immunohistochemistry for hTERT. (a, b) Positive cases (patients 3 and 58, respectively) demonstrate nuclear staining in mononuclear cells, and variably in osteoclast-like giant cells. (c) Area of so-called ‘benign fibrous histiocytoma’-like morphology with storiform spindle cells (patient 13) demonstrates nuclear staining in only a rare small lymphocyte.
Analysis of Outcome
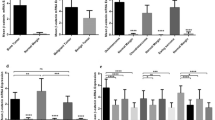
Using univariate (Kaplan–Meyer) survival analysis, nuclear staining with hTERT correlated with recurrence-free survival (P=0.02; hazard ratio 0.2, 95% confidence interval (CI) 0.05–0.8; Figure 3a) but not metastasis-free survival (P=0.8; hazard ratio 0.9, 95% CI 0.2–3.4; Figure 3b). Conversely, metastasis-free survival was significantly related to soft-tissue extension (P=0.003; hazard ratio 0.09, 95% CI 0.03–0.5; Figure 3d). While recurrence-free survival at the end of the study was 65% for patients with soft-tissue extension, compared with 73% for those without extension, this result did not reach statistical significance (P=0.3; hazard ratio 0.5, 95% CI 0.13–2.0; Figure 3c). Finally, the type of surgery, curettage or en bloc resection, did not correlate with recurrence-free survival (P=0.2; hazard ratio 0.45 95% CI 0.7–2.0) or metastasis-free survival (P=0.7; hazard ratio 0.7, 95% CI 0.1–4.0).
Survival statistics. Nuclear staining with hTERT correlates significantly with recurrence-free survival (a, P=0.02) but not metastasis-free survival (b, P=0.8). Conversely, the presence of soft-tissue extension at the time of first diagnosis does not correlate with recurrence-free survival (c, P=0.3) but does so with metastasis-free survival (d, P=0.003).
Discussion
Although telomerase enzyme activity measured using the TRAP assay has been regarded as the gold standard for measuring telomerase expression, some investigators have successfully used hTERT mRNA level36 or immunohistochemistry.23, 24, 25, 26, 27, 28, 29 In some cells, telomerase activity does not uniformly correlate with hTERT mRNA levels, suggesting a role for post-transcriptional modification in controlling enzyme activity.30 Nevertheless, a growing body of evidence suggests that immunohistochemistry is a reliable surrogate to technically more complex assays. Immunohistochemistry also allows detection of the specific population of hTERT-positive cells in a tumor comprised of a heterogeneous cell population, such as giant-cell tumor. In this study, we demonstrate that hTERT is present in the mononuclear cells and, to a lesser extent, the osteoclast-like giant cells, but not in the fibrous histiocytoma-like areas of giant-cell tumor. Furthermore, the study is the first to demonstrate that immunohistochemical staining for hTERT correlates with local recurrence in giant-cell tumor.
The mechanism by which hTERT expression contributes to recurrence in giant-cell tumor is not known. High telomerase activity may allow continued proliferation of tumor cells by maintaining telomere length during successive cell divisions. If so, our study suggests that the mechanisms of metastasis, which did not correlate with hTERT staining, may be unrelated to proliferation. These findings corroborate the results of Matsuo et al17 that telomerase activity was higher in giant-cell tumor that recurred, but not in those that metastasized. The results contrast with many metastatic carcinomas that display increased telomerase activity.18, 37, 38 Intriguingly, some have suggested that the so-called ‘metastatic’ lung nodules of giant-cell tumor represent implants of tumor emboli rather than true metastases.14, 39 This hypothesis is supported by the spontaneous regression of lung nodules in some patients.40
The incidence of metastasis in this study (16%) was higher than what has been generally reported. This finding raised concern that we unintentionally included higher-grade tumors, with concomitant higher risk of metastasis, in the series. However, no cases demonstrated high-grade nuclear features, atypical mitotic figures (including in the metastases) or radiographic features to suggest, for example, malignant giant-cell tumor or giant-cell-rich osteosarcoma. Furthermore, despite lack of systemic cytotoxic chemotherapy, all patients were alive at the end of the study, including patients who survived over 4 years after the diagnosis of metastases. These findings would be unusual if a high-grade sarcoma or other occult malignancy produced the pulmonary metastases. We do note that metastatic rate as high as in our series has been reported by others,41 and the variability may result from an element of selection bias. That is, patients with metastases, especially at the time of initial diagnosis, are more likely to be referred to tertiary care centers with multidisciplinary teams, which specialize in these complex cases.
Previous reports have suggested that the neoplastic cells of giant-cell tumor are primitive osteoblast precursors.12, 42 In giant-cell tumor, one might expect only this population of cells to express telomerase if these represent the transformed, immortalized cells. Thus, finding hTERT staining in the nuclei of presumably terminally differentiated osteoclast-like giant cells was unexpected. One explanation may stem from the mechanism of telomerase regulation. At least in cell-culture models, the hTERT promoter is activated by NF-kappa B.43 Additionally, the NF-kappa B-signaling pathway, through interactions between receptor of activated NF-kappa B (RANK) and its ligand (RANK-L), plays a crucial role in the recruitment and differentiation of the osteoclast-like cells from monocyte precursors in giant-cell tumor.12, 44, 45 Taken together, the above findings suggest that hTERT expression in the osteoclast-like cells of giant-cell tumor may be a manifestation of NF-kappa B activation in these cells, or their precursors, by RANK-L.
Outcome in giant-cell tumor has historically been difficult to predict from histological parameters. In fact, some authors have suggested that the type of surgery, simple curettage or resection, is a significant predictor of recurrence.2 En bloc resection, at least in theory, might be superior to eradicate microscopic residual disease. In the present study, we found no significant relationship between the type of initial surgery and recurrence or metastasis, despite the fact that most cases with soft-tissue extension were treated intralesionally with curettage (22/28, 79%) rather than en bloc resection. The lower than expected rate of local recurrence after intralesional procedures is explained by the fact that these patients were drawn from a single institution where improved preoperative imaging and more aggressive surgical technique have been used. Conversely, the higher than expected rate of recurrence in en bloc resection is explained by the comparatively larger and more aggressive tumors treated in this manner. That is to say, patients were not randomized as to surgical treatment method, and thus, biological factors, such as hTERT expression, may better explain recurrence-free survival.
In conclusion, hTERT is expressed in the mononuclear and, to a lesser extent, the multinucleated giant cells in giant-cell tumor of bone. Positive immunohistochemical staining for hTERT correlates inversely with recurrence-free survival, independent of soft-tissue extension or the type of surgery, and may be a useful prognostic marker or potential treatment target in patients with giant-cell tumor.
References
Fletcher CDM, Unni KK, Mertens F, (eds) Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, 2002, pp 310–312.
Goldenberg RR, Campbell CJ, Bonfiglio M . Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am 1970;52:619–664.
Campanacci M, Baldini N, Boriani S, et al. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106–114.
Cheng JC, Johnston JO . Giant cell tumor of bone. Prognosis and treatment of pulmonary metastases. Clin Orthop Relat Res 1997;338:205–214.
Scully SP, Mott MP, Temple HT, et al. Late recurrence of giant-cell tumor of bone. A report of four cases. J Bone Joint Surg Am 1994;76:1231–1233.
Hutter RV, Worcester Jr JN, Francis KC, et al. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer 1962;15:653–690.
Malek F, Krueger P, Hatmi ZN, et al. Local control of long bone giant cell tumour using curettage, burring and bone grafting without adjuvant therapy. Int Orthop 2006;30:495–498.
Sanerkin NG . Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer 1980;46:1641–1649.
Ling L, Klein MJ, Sissons HA, et al. Expression of Ia and monocyte–macrophage lineage antigens in giant cell tumor of bone and related lesions. Arch Pathol Lab Med 1988;112:65–69.
Aqel NM, Pringle JA, Horton MA . Cellular heterogeneity in giant cell tumour of bone (osteoclastoma): an immunohistological study of 16 cases. Histopathology 1988;13:675–685.
Goldring SR, Roelke MS, Petrison KK, et al. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest 1987;79:483–491.
Robinson D, Einhorn TA . Giant cell tumor of bone: a unique paradigm of stromal–hematopoietic cellular interactions. J Cell Biochem 1994;55:300–303.
O'Donnell RJ, Springfield DS, Motwani HK, et al. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am 1994;76:1827–1833.
Rock MG, Pritchard DJ, Unni KK . Metastases from histologically benign giant-cell tumor of bone. J Bone Joint Surg Am 1984;66:269–274.
McDonald DJ, Sim FH, McLeod RA, et al. Giant-cell tumor of bone. J Bone Joint Surg Am 1986;68:235–242.
Gitelis S, Mallin BA, Piasecki P, et al. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Joint Surg Am 1993;75:1648–1655.
Matsuo T, Hiyama E, Sugita T, et al. Telomerase activity in giant cell tumors of bone. Ann Surg Oncol 2007;14:2896–2902.
Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011–2015.
Shay JW, Zou Y, Hiyama E, et al. Telomerase and cancer. Hum Mol Genet 2001;10:677–685.
Shay JW, Bacchetti S . A survey of telomerase activity in human cancer. Eur J Cancer 1997;33:787–791.
Schwartz HS, Juliao SF, Sciadini MF, et al. Telomerase activity and oncogenesis in giant cell tumor of bone. Cancer 1995;75:1094–1099.
Morin GB . The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989;59:521–529.
Sabah M, Cummins R, Leader M, et al. Immunohistochemical detection of hTERT protein in soft tissue sarcomas: correlation with tumor grade. Appl Immunohistochem Mol Morphol 2006;14:198–202.
Martin JA, DeYoung BR, Gitelis S, et al. Telomerase reverse transcriptase subunit expression is associated with chondrosarcoma malignancy. Clin Orthop Relat Res 2004;426:117–124.
Aue G, Muralidhar B, Schwartz HS, et al. Telomerase activity in skeletal sarcomas. Ann Surg Oncol 1998;5:627–634.
Sangiorgi L, Gobbi GA, Lucarelli E, et al. Presence of telomerase activity in different musculoskeletal tumor histotypes and correlation with aggressiveness. Int J Cancer 2001;95:156–161.
Schneider-Stock R, Rys J, Jaeger V, et al. Prognostic significance of telomerase activity in soft tissue sarcomas. Int J Oncol 1999;15:775–780.
Tomoda R, Seto M, Tsumuki H, et al. Telomerase activity and human telomerase reverse transcriptase mRNA expression are correlated with clinical aggressiveness in soft tissue tumors. Cancer 2002;95:1127–1133.
Kleideiter E, Schwab M, Friedrich U, et al. Telomerase activity in cell lines of pediatric soft tissue sarcomas. Pediatr Res 2003;54:718–723.
Liu K, Schoonmaker MM, Levine BL, et al. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc Natl Acad Sci USA 1999;96:5147–5152.
Kido A, Schneider-Stock R, Hauptmann K, et al. Telomerase activity in benign bone tumors and tumor-like lesions. Pathol Res Pract 1999;195:753–757.
Bertoni F, Bacchini P, Staals EL . Malignancy in giant cell tumor of bone. Cancer 2003;97:2520–2529.
Nascimento AG, Huvos AG, Marcove RC . Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer 1979;44:1393–1402.
Horvai AE, Kramer MJ, O'Donnell R . Beta-catenin nuclear expression correlates with cyclin D1 expression in primary and metastatic synovial sarcoma: a tissue microarray study. Arch Pathol Lab Med 2006;130:792–798.
Sciot R, Dorfman H, Brys P, et al. Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions. A report from the CHAMP study group. Mod Pathol 2000;13:1206–1210.
Sanders RP, Drissi R, Billups CA, et al. Telomerase expression predicts unfavorable outcome in osteosarcoma. J Clin Oncol 2004;22:3790–3797.
Marchetti A, Bertacca G, Buttitta F, et al. Telomerase activity as a prognostic indicator in stage I non-small cell lung cancer. Clin Cancer Res 1999;5:2077–2081.
Tatsumoto N, Hiyama E, Murakami Y, et al. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin Cancer Res 2000;6:2696–2701.
Bertoni F, Present D, Enneking WF . Giant-cell tumor of bone with pulmonary metastases. J Bone Joint Surg Am 1985;67:890–900.
Siebenrock KA, Unni KK, Rock MG . Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br 1998;80:43–47.
Faisham WI, Zulmi W, Saim AH, et al. Pulmonary metastases of giant cell tumour of the bone. Med J Malaysia 2004;59 (Suppl F):78–81.
Oreffo RO, Marshall GJ, Kirchen M, et al. Characterization of a cell line derived from a human giant cell tumor that stimulates osteoclastic bone resorption. Clin Orthop Relat Res 1993;296:229–241.
Sinha-Datta U, Horikawa I, Michishita E, et al. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood 2004;104:2523–2531.
Roux S, Amazit L, Meduri G, et al. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol 2002;117:210–216.
Atkins GJ, Haynes DR, Graves SE, et al. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res 2000;15:640–649.
Acknowledgements
We thank Ms Loretta Chan and the UCSF Comprehensive Cancer Center Immunology Core for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Horvai, A., Kramer, M., Garcia, J. et al. Distribution and prognostic significance of human telomerase reverse transcriptase (hTERT) expression in giant-cell tumor of bone. Mod Pathol 21, 423–430 (2008). https://doi.org/10.1038/modpathol.3801015
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3801015