Abstract
The proliferation factor mitotic activity index is the strongest prognostic factor in early breast cancer, but it may lack reproducibility. We analyzed the prognostic value of phosphohistone H3, a marker of cells in late G2 and M phase, measuring highly standardized immunohistochemical nuclear phosphohistone H3 expression by subjective counts and digital image analysis. Expression was compared with classical clinico-pathologic prognostic variables and the mitotic activity index in 119 node-negative invasive breast cancers in patients less than 55 years old treated with adjuvant systemic chemotherapy with long-term follow-up (median 168 months). Nineteen patients (16%) developed distant metastases and 16 (13%) died. Strong phosphohistone H3 expression occurred preferentially in the peripheral growing front; counts were highly reproducible between observers (R=0.92) and highly consistent with digital image analysis (R=0.96). Phosphohistone H3 correlated (P<0.05) with tumor diameter, estrogen receptor, carcinoma grade, and mitotic activity index. Phosphohistone H3 values were systematically (80%) higher than the mitotic activity index. Receiver-operating curve analysis objectively showed that phosphohistone H3 <13 (n=53; 45% of all cases) vs phosphohistone H3≥13 (n=66; 55% of all cases) was the strongest prognostic threshold, with 20-year recurrence-free survival of distant metastases of 96 and 58%, respectively (P=0.0002, HR=9.6). Mitotic activity index was the second strongest prognostic variable (P=0.003, HR=3.9). In multivariate analysis, phosphohistone H3 <13 vs≥13 exceeded the prognostic value of the mitotic activity index. None of the other classical prognostic factors examined offered prognostic value additional to phosphohistone H3. Phosphohistone H3 is by far the strongest prognostic variable in early invasive node-negative breast cancer patients less than 55 years old with long-term follow-up.
Similar content being viewed by others
Main
The prognosis of patients with lymph node-negative breast cancer is relatively good (20–30% die from recurrent disease). Their improvement of survival with adjuvant systemic therapy is less substantial than that for lymph node-positive patients; the typical 15-year survival difference for treated and untreated patients is approximately 35% (relative) and 10% (absolute). Both the benefits and disadvantages of this 10% absolute improvement in survival must be considered when deliberating adjuvant systemic therapy.1
In women diagnosed with breast cancer, less than 70 years of age, proliferation markers such as the mitotic activity index (MAI) and thymidine labeling index have a greater prognostic value than do classical predictors.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 The MAI is a strong, widely available, easy to use, inexpensive, and highly reproducible prognostic variable.4, 6, 7, 13, 15, 16, 18 Notably, most genes constituting prognostic gene signatures are linked to proliferation.19, 20, 21 Moreover, in two independent studies, adjuvant chemotherapy was significantly beneficial to patients with rapidly proliferating tumors, but not patients with slowly proliferating tumors.22, 23 However, mitosis counts are not well liked by many pathologists, particularly due to issues with section quality and difficulty distinguishing mitoses. In addition, not all studies confirm the prognostic value of proliferation factors.24, 25 This may be due to differences in sample processing, methodological differences in MAI assessment, or inclusion of patients over 70 years of age (MAI is not prognostic in these patients17). Tissue processing is an unlikely source of disparity in most pathology laboratories, as mitoses are stable and robust with application of certain simple and widely used precautions.26, 27 A large multicenter study with strict adherence to well-validated protocol guidelines demonstrated good reproducibility of the MAI.28 However, it can never be fully guaranteed that reported MAI assessments have been performed following strict protocols. Moreover, for several reasons, including hypoxia, fixation delay, and suboptimal fixation, mitotic figures can become more condensed and thereby less easily identifiable.
Clearly, an accurate immunohistochemical proliferation marker would be very valuable. Unfortunately, most available immunohistochemical markers are much weaker predictors than the MAI.15 This may be due to the fact that mitotic figures reflect a very small time window in the cell cycle (ie, the M phase), whereas other markers such as Ki-67 stain cells in nearly all phases of the cell cycle. Many Ki-67-positive nuclei may not survive the cell cycle and are driven into apoptosis, thereby blurring the prognostic value of the Ki-67 index. However, mitoses are very late cell cycle markers, and once formed are reliable proliferative markers. Ideally, any substitute immunohistochemical proliferation marker should be expressed only in late G2 and M phase.
Recent studies demonstrate a tight correlation between phosphorylation of histone H3 (PPH3) and mitotic chromatin condensation; these studies used an antibody selective for the Ser-10 phosphorylated histone H3 (PPH3) NH2.29, 30, 31, 32 PPH3 expression has been characterized in human endometrium, colorectal cancers, ovarian serous adenocarcinomas, and meningiomas. In one small study of breast cancers, PPH3 expression was much less frequent than Ki67 expression, and there was a strong correlation between PPH3 expression and mitotic counts, suggesting that PPH3 counts can be used for grading of breast adenocarcinomas.33 However, the prognostic value of PPH3 in breast cancer has not yet been assessed.
In this study, we evaluated the prognostic value of PPH3 in a homogeneous group of node-negative invasive breast cancers from women less than 55 years of age at diagnosis, treated with adjuvant systemic chemotherapy. Use of prognostically homogeneous breast cancers is essential to avoid selection bias. For example, mixing node-negative and -positive cancers or young and old patients can seriously blur the value of important prognostic features, such as proliferation.17 We quantitatively assessed immunohistochemical PPH3 expression by both subjective counts and quantitative digital image analysis (DIA).
Materials and methods
Patients
The study was approved by the Regional Ethics Committee, the Norwegian Social Science Data Service, and the Norwegian Data Inspectorate. Paraffin-embedded material from 1169 breast tumor patients treated between 1978 and 1994 was provided by the Department of Pathology at the Stavanger University Hospital (Stavanger, Norway). Of these patients, 294 were excluded for the following reasons: carcinoma in situ (n=81), extensive carcinoma in situ with a microinvasive component <1 mm that was ineligible for MAI evaluation (n=88), history of breast cancer (n=11), recurrence within 6 months of follow-up (n=19), <6-month follow-up (41), Paget's disease (n=18), bilateral breast cancer (n=23), male gender (n=2), and rare, noncancerous breast malignancies (n=11). Material was not available for 14 patients, and 29 were lost to follow-up. Thus, material from 832 patients was available for analysis. Of the 832 patients, 442 were lymph node negative, and 186 also met the age criterion (<55 years). Following the protocol of the Norwegian Breast Cancer Group, 125 of the 186 eligible lymph node-negative patients received systemic adjuvant cyclophosphamide, methotrexate, 5-fluorouracil chemotherapy. Insufficient tissue was available from six of these patients, leaving 119 patients with assessable material. All patients were treated with modified radical mastectomy or breast-conserving therapy (with adequate lymph node dissection, at least 10 nodes examined). Locoregional radiotherapy was administered to patients who underwent breast-conserving therapy or had medially localized tumors.
Pathology
The post-surgical size of the tumor was measured in fresh specimens. Tumors were cut into 0.5-cm slices, fixed in 4% buffered formaldehyde, and embedded in paraffin. Paraffin sections were cut into 4-μm sections and stained with hematoxylin–eosin (H&E). Histological type was assessed according to World Health Organization criteria.34 Grade (Grade 1=3, 4, or 5; Grade 2=6 or 7; Grade 3=8 or 9) was assessed according to the Nottingham modification,35, 36 calculated as the sum of tubular formation (>75%=1, 10–75%=2, and <10%=3), nuclear atypia (mild=1, moderate=2, and marked=3), and MAI class (0–5=1, 6–10=2, and >10=3). The MAI was assessed as described elsewhere.37 Briefly, all unambiguous mitoses were counted in 10 consecutive neighboring fields of vision (FOV) in the most cell-dense area (1.59 mm2 at specimen level), usually in the peripheral growing zone. The MAI is reproducible and is insensitive to variations in tissue processing.26, 27, 28
Immunohistochemistry
Antigen retrieval and antibody dilution were optimized prior to study onset. To ensure uniform handling of samples, all sections were processed simultaneously. Paraffin sections adjacent to the H&E sections used for assessment of MAI and histology were mounted onto silanized slides (#S3003; Dako, Glostrup, Denmark) and dried overnight at 37°C followed by 1 h at 60°C Sections were deparaffinized in xylene and rehydrated in decreasing concentrations of alcohol. Antigen was retrieved with a highly stabilized retrieval system (ImmunoPrep, Instrumec, Oslo, Norway) using 10 mM TRIS/1 mM EDTA (pH 9.0) as the retrieval buffer. Sections were heated for 3 min at 110°C followed by 10 min at 95°C and cooled to 20°C. Rabbit polyclonal anti-phosphohistone H3 (ser 10) (Upstate #06–570; Lake Placid, NY, USA) was used at a dilution of 1:1500, and the sections were incubated for 60 min at 22°C Dako antibody diluent (S0809) was used. Endogenous peroxidase activity was blocked with a peroxidase blocking reagent (S2001; Dako) for 10 min. The immune complex was visualized with the Dako REAL EnVision Detection System, Peroxidase/DAB, Rabbit/Mouse (K5007; Dako). Sections were incubated with EnVision/HRP, Rabbit/Mouse for 30 min and diaminobenzidine (DAB+) chromogen for 10 min. The sections were counterstained with hematoxylin, dehydrated, and mounted. All steps were performed using Dako Autostainer and TBS (S1968; Dako) with 0.05% Tween 20 as wash buffer. Normal breast tissue from the same section was used as a control.
Quantification of PPH3-Positive Nuclei
The PPH3 index was assessed as follows. Using the same counting protocol as for the MAI, two independent pathologists counted the number of PPH3-positive objects (nuclei and mitoses) in 10 adjacent FOV, with a × 40 objective, as described above, in the same invasive epithelial portions of the most PPH3-positive areas. Nuclei with fine granular PPH3 staining were not counted, as these cells are not in the G2 phase.33 PPH3-rich areas are usually localized in the periphery (ie, growing zone) of the cancers. If the counts of two observers differed by more than three figures, the count was repeated with a multi-head microscope and a consensus score was obtained.
Automated Digital Image Analysis
In addition to performing subjective counts, PPH3 expression was evaluated using the Image-J DIA program (National Institutes of Health, Washington, USA; http://rsb.info.nih.gov/ij/) in areas subjectively determined to have the highest frequency of PPH3-positive nuclei, almost exclusively located in the peripheral growing zone of the tumor. Six FOV were selected with a × 20 objective (Numeric Aperture 0.40; the total area of the five FOV at the specimen level was 1.803 mm2) and digitized using a Scion Image (CFW-1312C) color CCD camera mounted on a Leica LM DM microscope with a CTRMIC control unit (Leica, Wetzlar, Germany). To ensure stable image quality, the camera and microscope lamp system were supplied by a UPS voltage stabilizer (guaranteed 230±1 V), and the microscope aperture and light settings were automatically controlled by the Leica DM SDK4.1/MIC software. Before each image capturing session, the system was calibrated with an optical density calibration slide (PRESS-PRO21; Leica, Cambridge, UK).
Using built-in ImageJ program functions, we created a macro that automatically detects and counts immunohistochemically stained mitotic cells. Figure 1 illustrates the image processing steps. Briefly, a combination of color deconvolution and threshold was used to create a binary image containing all objects immunohistochemically stained with di-aminobenzidine. Objects smaller than mitotic figures were removed by a size filter. The remaining objects were dilated to fuse the chromatin structures of cells in anaphase or telophase into one object. All remaining objects were counted, and the number of objects per 1.59 mm2 was calculated. Counted objects were encircled in the original image, allowing visual inspection of the counted mitotic cells. Apart from the selection of the five FOV, the DIA procedure and all calculations were fully automated.
Typical images for digital image analysis to automatically measure the PPH3-index. (a) Original image. (b) Color deconvoluted hematoxylin staining and (c) immunohistochemical staining. (d) Binary image of (c) after thresholding. (e) Same as (d) but small objects are removed and remaining objects are dilated to fuse metaphase chromosomes into one object. (f) Original image with all PPH3-positive objects indicated by a yellow circle for visual inspection of the automatically selected and counted objects.
Reproducibility of the PPH3 measurements was evaluated 2 and 4 weeks after the first assessment in 10 randomly selected cases, by different operators, who selected the five FOV using the selection criteria described above without knowledge of where the FOV were selected in the first or second assessments. The inter-observer results were highly reproducible (R=0.96–0.99).
Data Analysis
Correlations were calculated using Spearman's and κ tests. For survival analysis, the main endpoints were distant metastases recurrence and overall distance metastases-related survival. To determine the probability that patients would remain free of distant metastases, we defined recurrence as any first recurrence at a distant site. Patients were censored from the date of the last follow-up visit for death from causes other than breast cancer, local or regional recurrences, and the development of a second, primary cancer, including contralateral breast cancer. If a patient's status during follow-up indicated a confirmed metastasis without a recurrence date, the follow-up visit date was used. Age, time to first recurrence, and survival time were calculated relative to the primary diagnosis date. For the MAI, three sets of previously established prognostic thresholds (<6, 6–10, ≥11,36 <10 vs ≥10; and <3, 3–9, and ≥1038) were examined. There was a continuous spectrum of PPH3 values from very few to many positive nuclei. For survival analysis these continuous data were divided into different subgroups, using a threshold value. We used receiver-operating curves to identify the objectively optimal threshold. Kaplan–Meier survival curves were constructed, and between-group differences were tested using the log–rank test. The relative importance of potential prognostic variables was tested using Cox-proportional hazard analysis and expressed as a hazard ratio (HR) with a 95% confidence interval (CI).
Results
H3 Staining Patterns
Application of the antigen retrieval and PPH3 staining method to early breast cancers revealed discrete, crisp, contrast-rich, and strong staining of mitoses and a small fraction of intact nuclei (Figure 2) at a dilution of 1:1500. Occasionally, minimal fine granular staining occurred; such nuclei were not counted. There was no cytoplasmic staining. PHH3 was easily identified, even in histologically suboptimal sections (Figure 3).
Node-negative breast cancer. Examples of mitotic figures in poor (a), moderate (b), and good quality (c) H&E sections. (d–f) Histone-3 nuclear staining from the same area of sections adjacent to the H&E sections. Mitoses are marked with arrows. Note the contrast-rich PPH3 staining of all prophase and metaphase mitotic cells, some showing chromatin clustering and condensation, which occur in early prophase just before metaphase.
Correlations of PPH3 Staining and Other Characteristics
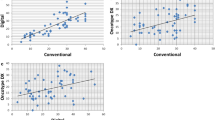
There was a continuous spectrum of PPH3 values between 0 and 200, and no clear peaks were detected in the frequency histogram. Using median, tertiles, quartiles, and quintiles of PPH3 DIA values and receiver-operating curves analysis (Figure 4) we determined that a value ≥13 was a reproducible threshold and also the strongest predictor of survival (see below). In 53 of 119 patients (45%) the PPH3 was less than 13 and in 66 of 119 patients (55%) it was greater than or equal to 13. There was a strong correlation between the automated DIA PPH3 measurements and the subjective counts of PPH3 (R=0.96), whereas the correlation between DIA PPH3 measurements and MAI was also significant but with a wider variation (R=0.82) (Figure 5). This is probably due to the highly contrast-rich staining pattern of PPH3, which makes identifying positively stained objects easier for a human observer than counting mitoses in an H&E section.
Table 1 shows the correlations of the dichotomized subjective ratings of the PPH3 index and clinico-pathologic characteristics. A high PPH3 index (≥13) correlated with large tumor diameter >2 cm), negative estrogen receptor and progesterone receptor, high carcinoma grade, high Mitotic Activity Index, low tubular formation (<10%), and marked nuclear atypia, but not with age. A high PPH3 index most strongly correlated with the MAI.
Prognostic Evaluation
Median duration of follow-up was 168 months (range 10–339). Table 2 shows the prognostic value of the different clinico-pathologic features (only the most prognostic results are shown). Of the classic features, tumor diameter (P=0.05), carcinoma grade (P=0.006), and MAI <10 vs ≥10 (P=0.003, HR=3.9) were strongly prognostic. Multivariate survival analysis of tubular formation, nuclear atypia, and MAI (categories: 0–5, 6–10, and >10), showed that MAI was the only independent predictor. After inclusion of the MAI, P-values for tubular formation and nuclear atypia were 0.41 and 0.78, respectively. MAI <10 vs ≥10 was the strongest prognostic threshold. None of the other clinico-pathologic features added significantly to the prognostic value of the MAI. However, the prognostic value of the PPH3 index greatly exceeded that of all other features, and none of them did add to its prognostic value (Figure 6). Forty-five percent of the women with early breast cancer showed limited PPH3 expression (<13) and these patients had an excellent prognosis for long-term disease-specific survival (96%, both at 10- and 20-year follow-up, in contrast to women with PPH3 expression ≥13 (80 and 58% survival at 10- and 20-year follow-up, respectively) (P<0.0002, HR=9.6).
Discussion
The current study shows that nuclear PPH3 expression is the strongest prognostic factor for node-negative breast cancer patients less than 55 years of age treated with systemic adjuvant chemotherapy. PPH3 expression greatly exceeded the prognostic value of other classical prognostic factors, including the MAI, which until now was considered the strongest prognostic factor for this subgroup. Development of an optimal balance between a favorable or improved prognosis and the toxic side effects of chemotherapy for these patients is a major goal of many research projects. Thus, our demonstration of the strong prognostic value of PPH3 is of special clinical importance. Moreover, our findings demonstrate another advantage of the PPH3 method, which is its production of strong, robust, and contrast-rich staining in standard immunohistochemical DAB-stained sections, which prevents false-positive counts.
It is worthy to note that in multivariate analysis, the prognostic significance of both PPH3 index and MAI greatly exceeded that of tubular formation and nuclear atypia.36 The current results, together with previous findings, clearly establish that PPH3 counts can replace any of the three constituents of carcinoma grading. It is also interesting that PPH3 was much stronger prognostically than estrogen and progesterone receptor. This casts doubt on the use of steroid receptors as primary prognostic classifiers in node-negative breast cancer patients less than 55 years of age. In older patients (>70 years), presence of estrogen receptors is a stronger predictor than proliferation, and therefore can be used as a primary prognostic variable.17 Interestingly, and consistent with our findings, prognostic gene signatures mostly incorporate proliferative factors.19, 21, 31
In spite of the strong prognostic value of PPH3, and the excellent prognosis of patients with a low PPH3 index, more than 50% of patients with high PPH3 proliferation survived without signs of distant metastases. This suggests that other factors related to invasion and metastasis may have prognostic value additional to PPH3. These may include hypoxia-related factors,2 as well as vascular tumor factors,39 PAI-1/UpA,40 and bone marrow micrometastases.41 It is important to investigate the prognostic value of these factors in comparison to PPH3.
In conclusion, nuclear PPH3 expression is the strongest prognostic variable in operable lymph node-negative breast cancer patients less than 55 years of age. It remains to be assessed if the same also holds for older node-negative and -positive patients.
References
Early Breast Cancer Trialists' Collaborative Group (EBCTG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–1717.
Baak JP, Colpaert CG, van Diest PJ, et al. Multivariate prognostic evaluation of the activity index and fibrotic focus in node-negative invasive breast cancers. Eur J Cancer 2005;41:2093–2101.
Baak JP, van Diest PJ, Voorhorst FJ, et al. Prospective multicenter validation of the independent prognostic value of the mitotic activity index in lymph node-negative breast cancer patients younger than 55 years. J Clin Oncol 2005;23:5993–6001.
Baak JPA, van Dop H, Kurver PHJ, et al. The value of morphometry to classic prognostic variables in breast cancer. Cancer 1985;56:374–382.
Bergers E, Baak JP, van Diest PJ, et al. Prognostic implications of different cell cycle analysis models of flow cytometric DNA histograms of 1301 breast cancer patients: results from the Multicenter Morphometric Mammary Carcinoma Project. Int J Cancer 1997;74:260–269.
Groenendijk RP, Bult P, Noppen CM, et al. Mitotic activity index in interval breast cancers. Eur J Surg Oncol 2003;29:29–31.
Manders P, Bult P, Sweep CG, et al. The prognostic value of the mitotic activity index in patients with primary breast cancer who were not treated with adjuvant systemic therapy. Breast Cancer Res Treat 2003;77:77–84.
Meyer JS, Alvarez C, Milikowski C, et al. Breast carcinoma malignancy grading by Bloom–Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol 2005;18:1067–1078.
Sigurdsson H, Baldetorp B, Borg A, et al. Indicators of prognosis in node-negative breast cancer. N Engl J Med 1990;322:1045–1053.
Silvestrini R, Daidone MG, Di Fronzo G, et al. Prognostic implication of labeling index versus estrogen receptors and tumor size in node-negative breast cancer. Breast Cancer Res Treat 1986;7:161–169.
Thor AD, Liu S, Moore II DH, et al. Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol 1999;17:470–477.
Tosi P, Luzi P, Sforza V, et al. Correlation between morphometrical parameters and disease-free survival in ductal breast cancer treated only by surgery. Appl Pathol 1986;4:33–42.
Uyterlinde AM, Baak JP, Schipper NW, et al. Prognostic value of morphometric and DNA flow-cytometry features of invasive breast cancers detected by population screening: comparison with control group of hospital patients. Int J Cancer 1991;48:173–181.
van der Linden JC, Baak JP, Lindeman J, et al. Prospective evaluation of prognostic value of morphometry in patients with primary breast cancer. J Clin Pathol 1987;40:302–306.
van Diest PJ, van der Wall E, Baak JP . Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol 2004;57:675–681.
Volpi A, Bacci F, Paradiso A, et al. Prognostic relevance of histological grade and its components in node-negative breast cancer patients. Mod Pathol 2004;17:1038–1044.
Baak JP, van Diest PJ, Voorhorst FJ, et al. The prognostic value of proliferation in lymph-node-negative breast cancer patients is age dependent. Eur J Cancer 2007;43:527–535.
Fleege JC, Diest PJ, Baak JPA . Reliability of quantitative pathological assessments: counting mitoses. In: Baak JPA (ed). Manual of Quantitative Pathology in Cancer Diagnosis and Prognosis. Springer: New York, 1991, pp 169–172.
Sims AH, Ong KR, Howell A, et al. High-throughput genomic technology in research and clinical management of exploiting the potential of gene expression profiling: is it ready for the clinic? Breast Cancer Res 2006;8:214.
Dai H, van't Veer L, Lamb J, et al. A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Res 2005;65:4059–4066.
Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 2006;98:262–272.
Amadori D, Nanni O, Marangolo M, et al. Disease-free survival advantage of adjuvant cyclophosphamide, methotrexate, and fluorouracil in patients with node-negative, rapidly proliferating breast cancer: a randomized multicenter study. J Clin Oncol 2000;18:3125–3134.
Andre F, Khalil A, Slimane K, et al. Mitotic index and benefit of adjuvant anthracycline-based chemotherapy in patients with early breast cancer. J Clin Oncol 2005;23:2996–3000.
Fiets WE, Bellot FE, Struikmans H, et al. Prognostic value of mitotic counts in axillary node negative breast cancer patients with predominantly well-differentiated tumours. Eur J Surg Oncol 2005;31:128–133.
Westenend PJ, Meurs CJ, Damhuis RA . Tumour size and vascular invasion predict distant metastasis in stage I breast cancer. Grade distinguishes early and late metastasis. J Clin Pathol 2005;58:196–201.
Jannink I, Risberg B, Van Diest PJ, et al. Heterogeneity of mitotic activity in breast cancer. Histopathology 1996;29:421–428.
Bergers E, Jannink I, van Diest PI, et al. The influence of fixation delay on mitotic activity and flow cytometric cell cycle variables. Hum Pathol 1997;28:95–100.
van Diest PJ, Baak JP, Matze-Cok P, et al. Reproducibility of mitosis counting in 2469 breast cancer specimens: results from the Multicenter Morphometric Mammary Carcinoma Project. Hum Pathol 1992;23:603–607.
Brenner RM, Slayden OD, Rodgers WH, et al. Immunohistochemical assessment of mitotic activity with an antibody to phosphorylated histone PPH3 in the macaque and human endometrium. Hum Reprod 2003;18:1185–1193.
Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 phase and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997;106:348–360.
Juan G, Traganos F, James WM, et al. Histone PPH3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry 1998;32:71–77.
Juan G, Traganos F, Darzynkiewicz Z . Histone PPH3 phosphorylation in human monocytes and during HL-60 cell differentiation. Exp Cell Res 1999;246:212–220.
Bossard C, Jarry A, Colombeix C, et al. PPH3-based a phosphohistone PPH3 labelling for histoprognostic grading of breast adenocarcinomas and computer-assisted determination of mitotic index. J Clin Pathol 2006;59:706–710.
Tavassoli FA, Devilee P (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press: Lyon, 2003, pp 9–112.
Eppenberger-Castori S, Moore Jr DH, Thor AD, et al. Age-associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol 2002;34:1318–1330.
Sloane JP, Amendoeira I, Apostolikas N, et al. Consistency achieved by 23 European pathologists from 12 countries in diagnosing breast disease and reporting prognostic features of carcinomas. European Commission Working Group on Breast Screening Pathology. Virchows Arch 1999;434:3–10.
Baak JP, van Diest PJ, Ariens AT, et al. The multicenter morphometric mammary carcinoma project (MMMMCP). A nationwide prospective study on reproducibility and prognostic power of routine quantitative assessments in The Netherlands. Pathol Res Pract 1989;185:664–670.
Baak JP, van Diest PJ, Voorhorst FJ, et al. Prospective multicenter validation of the independent prognostic value of the mitotic activity index in lymph node-negative breast cancer patients younger than 55 years. J Clin Oncol 2005;23:5993–6001.
de Jong JS, van Diest PJ, Baak JP . Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology 2000;36:306–312.
Janicke F, Prechtl A, Thomssen C, et al. German N0 Study Group. Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J Natl Cancer Inst 2001;93:913–920.
Farmen RK, Nordgard O, Gilje B, et al. Bone marrow cytokeratin 19 mRNA level is an independent predictor of relapse-free survival in operable breast cancer patients. Breast Cancer Res Treat 2007; May 10 [E-pub ahead of print], PMID: 17492378.
Acknowledgements
This work was supported by Grant 911108 from Helse Vest. Grant 2007 from Folke Hermansen Foundation and Grant 06–347 from the Stichting Bevordering Diagnostiche Morfometrie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
Skaland, I., Janssen, E., Gudlaugsson, E. et al. Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol 20, 1307–1315 (2007). https://doi.org/10.1038/modpathol.3800972
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800972
Keywords
This article is cited by
-
Rebelled epigenome: histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy
Clinical Epigenetics (2020)
-
Dinaciclib, a cyclin-dependent kinase inhibitor, suppresses cholangiocarcinoma growth by targeting CDK2/5/9
Scientific Reports (2020)
-
The Effects of Anti-TGF-β1 on Epithelial–Mesenchymal Transition in the Pathogenesis of Adenomyosis
Reproductive Sciences (2020)
-
Influence of pre-operative oral carbohydrate loading vs. standard fasting on tumor proliferation and clinical outcome in breast cancer patients ─ a randomized trial
BMC Cancer (2019)
-
Phosphohistone H3 (PHH3) as a surrogate of mitotic figure count for grading in meningiomas: a comparison of PHH3 (S10) versus PHH3 (S28) antibodies
Virchows Archiv (2019)