Abstract
Synovial sarcoma accounts for between 6 and 10% of childhood sarcomas and histological diagnosis can be challenging, even for experienced pathologists. Several other tumors enter the differential diagnosis, including malignant peripheral nerve sheath tumor, Ewing sarcoma/primitive neuroectodermal tumor and undifferentiated sarcoma. Several recent reports utilizing expression array techniques have documented expression of the MYCN oncogene in synovial sarcoma. In order to more fully investigate this finding, a series of 12 synovial sarcomas and 29 other sarcomas (four malignant peripheral nerve sheath tumors, 15 Ewing sarcoma/primitive neuroectodermal tumors, 10 undifferentiated sarcomas) were examined for MYCN expression and gene amplification. By RT-PCR, nine of 12 synovial sarcomas (75%) expressed MYCN. Five synovial sarcomas (42%) expressed MYCN at high levels. Of the other sarcomas, one malignant peripheral nerve sheath tumor (25%) and five Ewing sarcoma/primitive neuroectodermal tumors (33%) expressed MYCN at low levels, and all other cases were negative for MYCN. None of the synovial sarcomas had genomic amplification, suggesting that high MYCN expression levels resulted from epigenetic phenomena. Examination of selected downstream targets of MYCN in synovial sarcoma revealed expression of MCM7 (minichromosome maintenance protein 7) in all synovial sarcomas, and expression of nestin (n=10; 83%), ID2 (inhibitor of DNA binding protein 2) (n=6; 50%) and MRP1 (multidrug resistance protein 1) (n=1; 8%) in a subset of synovial sarcomas. Expression of downstream targets did not correlate with expression of MYCN. Neither MYCN nor expression of downstream targets significantly correlated with metastases at presentation, progression-free survival or overall survival in this small series. In summary, high levels of MYCN expression was useful for distinguishing synovial sarcoma from other childhood-spindled cell sarcomas with specificity and sensitivity of 100 and 42%, respectively, in this series. The clinical and biological significance of this finding deserves further study.
Similar content being viewed by others
Main
Synovial sarcoma is a soft tissue tumor of uncertain mesenchymal cell origin. It accounts for 2–6% of sarcomas in childhood1 and up to 10% of sarcomas in late childhood and early adulthood,2 with most cases occurring between the ages of 15 and 35 years.2 The most common sites of occurrence are the extremities, usually around joints, with smaller numbers occurring in the head and neck, retroperitoneum and mediastinum.1, 2, 3 Histologically, there are four major types; biphasic, monophasic spindled, monophasic epithelioid and poorly differentiated, with the former two being the most common.1, 2, 4 Synovial sarcoma is associated with a specific balanced translocation t(X:18)(p11.2;q11.2) in more than 90% of cases.4, 5, 6 The rearrangement fuses the SYT gene on chromosome 18q11.2 to one of the SSX gene family members (SSX1, SSX2 or SSX4) located on Xp11.2.7, 8, 9, 10 Prognostic indicators of synovial sarcoma include age, size and morphology,4 as well as molecular genetics, with SSX2 translocations associated with a better outcome.11
The diagnosis of synovial sarcoma can be difficult to make, especially for the monophasic-spindled and poorly differentiated variants. The histological differential diagnoses include other childhood sarcomas, namely malignant peripheral nerve sheath tumor, Ewing sarcoma/primitive neuroectodermal tumor and undifferentiated sarcoma.12, 13 Malignant peripheral nerve sheath tumor can be particularly difficult to distinguish from synovial sarcoma, as they share several morphological, immunohistochemical and ultrastructural features.13, 14, 15 Some authors have even reported the rare occurrence of a t(X;18) translocation in malignant peripheral nerve sheath tumor,16 adding further difficulty in differentiating these two tumors. Thus, markers specifically expressed in either one tumor or the other would be extremely useful.
MYCN is a member of the MYC family of oncogenes, which also include CMYC and LMYC. Amplification of the MYCN gene is associated with advanced tumor stage, tumor progression and poor outcome in neuroblastomas.17 Several downstream targets of MYCN have been described and include the genes encoding the multidrug resistance protein 1 (MRP1),18 the minichromosome maintenance protein 7 (MCM7),19 the inhibitor of DNA binding protein 2 (ID2)20 and the intermediate filament nestin.21
Amplification and overexpression of MYCN has been described in tumors other than neuroblastoma, including alveolar rhabdomyosarcoma,22 astrocytoma,23 medulloblastoma,24 retinoblastoma,25 Wilms tumor26 and breast carcinoma.27 Two recent studies utilizing cDNA expression array analysis documented MYCN expression in synovial sarcoma.28, 29 With respect to malignant peripheral nerve sheath tumor, one study has documented MYCN expression,29 whereas others have not.30, 31 MYCN expression has not been reported in Ewing sarcoma/primitive neuroectodermal tumor.32, 33 In order to investigate the potential diagnostic usefulness of these findings, the current study examined the expression pattern of MYCN in a series of 12 synovial sarcomas and 29 other pediatric sarcomas by RT-PCR. In addition, the status of MYCN gene amplification and expression of downstream targets of MYCN was assessed in synovial sarcoma.
Materials and methods
Tumor Selection
Twelve cases of synovial sarcoma diagnosed between 1990 and 2004 were selected from the files of the Hospital for Sick Children, Toronto. All tumors were positive for the SYT/SSX fusion transcript indicative of the t(X;18)(p11.2;q11.2) chromosomal rearrangement. A series of other sarcomas entering the differential diagnosis of synovial sarcomas were also tested and included four malignant peripheral nerve sheath tumors (S100 positive, t(X;18) negative), 15 Ewing sarcoma/primitive neuroectodermal tumors (CD99 positive, EWS-rearrangement positive) and 10 undifferentiated sarcomas (see Somers et al12 for selection criteria). A series of neuroblastomas diagnosed during the same time period were used as a control group. The study design and implementation were approved by the Hospital for Sick Children Research Ethics Board.
RT-PCR for SYT/SSX Fusion Transcripts and MYCN and MRP1 Gene Expression
RNA was extracted from fresh snap-frozen tissue using routine Trizol-based methods. RT-PCR for the SYT/SSX1 and SYT/SSX2 fusion transcripts was performed according to previously published protocols.34 Semiquantitative RT-PCR for MYCN gene expression was performed using MYCN primers (MYCN forward 5′-CGA CCA CAA GGC CCT CAG TA-3′ and MYCN reverse 5′-CAG CCT TGG TGT TGG AGG AG-3′) and PBGD primers (PBGD sense 5′-CAT GTC TGG TAA CGG CAA TGC GGC TGC-3′ and PBGD antisense 5′-GAA CTC CAG ATG CGG GAA CTT TC-3′). The expression levels for MYCN were interpreted as high (similar to the level of expression in the neuroblastoma cell line NUB-7), low (similar to the level of expression in neuroblastoma cell line SK-N SH) or negative (normal tonsil RNA). Semiquantitative RT-PCR for MRP1 gene expression was performed using MRP1 primers (MRP1 forward 5′-TCT CTC CCG ACA TGA CCG AGG-3′ and MRP1 reverse 5′-CCA GGA ATA TGC CCC GAC TTC-3′) and PBGD primers as for the MYCN assay (see above). Expression levels of MRP1 were interpreted as high (similar to expression IMR32 cell line) or low (similar to expression of RNA from tonsil).
PCR for MYCN Amplification
DNA was extracted from fresh snap-frozen tissue using routine methods. Primers and conditions for semiquantitative PCR amplification of the MYCN gene were performed according to previously published protocols.35
Chromogenic In Situ Hybridization for MYCN Gene Amplification
Chromogenic in situ hybridization (CISH) was performed using the Spotlight® CISH polymer detection kit (Zymed Laboratories, San Francisco, CA, USA) using the digoxigenin-labeled Spotlight® N-MYC probe (Zymed Laboratories) as described previously.36 Scoring of the histological sections for amplification was performed according to previously published criteria.36 Briefly, amplification was defined as greater than 10 signals per tumor cell nucleus, or the presence of homogenously stained regions within tumor cell nuclei.
Immunohistochemistry for ID2, MCM7 and Nestin
Immunohistochemical analyses were performed on 4-μm thick sections using the Ventana DAB kit as per the manufacturer's instructions (Ventana Medical Systems, Tucson, AZ, USA). Antibodies tested were against ID2 (1:250, Santa Cruz Biotechnology, Santa Cruz, CA, USA), MCM7 (1:100, Santa Cruz) and nestin (1:200, Chemicon International, Temecula, CA, USA). Immunohistochemical stains were considered either positive or negative using the following scoring system. The sections were scored as per previously published criteria.12 Briefly, scores for intensity were 1 for low, 2 for moderate and 3 for high, with the positive control used as the standard for high intensity. The distribution of staining was scored as 1 for <10% of cells positive, 2 for 11–50% of cells positive and 3 for >50% of cells positive. A combined score of 4 or more was considered positive; <4 was considered negative.
Clinical Data
The material reviewed included operative reports (to identify extent of tumor resection at diagnosis), medical charts and the Hematology/Oncology database at the Hospital for Sick Children. Clinical parameters obtained were progression-free survival, the presence or absence of metastases at presentation and overall survival. Progression-free survival was defined as absence of clinical and radiological recurrence at the primary site up to the time of most recent follow-up. Recurrence was defined as relapsed disease occurring after documented disease remission. Progression-free survival and overall survival were calculated from the start of treatment to the time of recurrence or death from any cause, respectively.
Statistical Analyses
Non-parametric data were compared using Fisher's exact test. For each clinical and morphological parameter, the association with progression-free survival and overall survival was characterized by univariate analysis using the Kaplan–Meier method and log-rank test. Statistical analyses were performed using SPSS version 13.0 software. Significance was defined as P<0.05.
Results
Tumor Histology and RT-PCR for t(X;18)
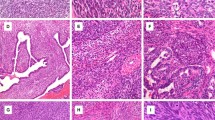
Twelve synovial sarcomas from nine male subjects and three female subjects were included in the study. Of the 12 synovial sarcomas, four had biphasic morphology, seven had monophasic-spindled morphology and one was poorly differentiated (Figure 1, Table 1). All tumors were positive for the characteristic SYT/SSX fusion transcript.
MYCN Gene Expression and Amplification in Pediatric Sarcomas
RNA was extracted from 12 synovial sarcomas. Nine of the 12 synovial sarcomas (75%) showed some expression of MYCN, with five (42%; two biphasic, two monophasic, one poorly differentiated) showing high levels of expression and four showing low levels of expression (Table 1). The synovial sarcomas with overexpression had expression levels comparable to that seen in neuroblastoma controls with MYCN gene amplification (Figure 2). High levels of MYCN expression were not associated with a specific histologic subtype (P=0.8485, Fisher's exact test). Of the other sarcomas tested, 5/15 Ewing sarcoma/primitive neuroectodermal tumors, 0/10 undifferentiated sarcomas and 1/4 malignant peripheral nerve sheath tumors expressed MYCN. All of the non-synovial sarcomas expressed MYCN at low levels. Thus, high levels of MYCN expression was useful for distinguishing synovial sarcoma from other childhood-spindled cell sarcomas with specificity and sensitivity of 100 and 42%, respectively, in this series (Table 2).
Semiquantitative RT-PCR analysis of the MYCN gene expression using RNA extracted from samples of synovial sarcoma (SS-1, patient 1; SS-2, patient 2). NVB-7, control for high MYCN expression; tonsil, control for negative MYCN expression; SK-N-SH, control for low MYCN expression; water control; SS-1 and SS-2, synovial sarcoma samples exhibiting high levels of MYCN expression.
MYCN gene amplification in the series of synovial sarcomas was tested for by a combination of CISH (all cases) and PCR (four cases). None of the synovial sarcomas showed amplification of the MYCN gene by CISH (Figure 3) or PCR.
CISH for the MYCN gene showing one to two signals per nucleus, consistent with diploidy in paraffin sections (a). Focal aneuploidy was seen in some sections as scattered nuclei with greater than two signals (b), however, amplification (>10 signals in the majority of tumor cells) was not present. Hematoxylin counterstain, × 600.
MRP1 Expression by RT-PCR in Synovial Sarcoma
Of the 12 synovial sarcomas, only one tumor expressed MRP in the low-mid range; this was associated with high levels of MYCN expression. All other tumors had negligible levels of expression.
ID2, MCM7 and Nestin Expression by Immunohistochemistry in Synovial Sarcoma
Examination of downstream targets of MYCN in synovial sarcomas revealed expression of MCM7 in all cases of synovial sarcoma. Nestin was expressed in 10 cases of synovial sarcoma (83%) and ID2 in six cases (50%) (Figure 4). Expression of downstream targets did not significantly correlate with high levels of MYCN expression; however, expression of MYCN at either low or high levels was associated with a trend toward expression of ID2 (P=0.0909, Fisher's exact test) (Table 1).
Prognostic Impact in Synovial Sarcoma
Expression of MYCN, MRP1, MCM7, ID2 or nestin was not significantly associated with metastases at presentation, local recurrence or survival. However, tumors that recurred locally were all ID2-positive (3/3, 100%), compared with 3/9 (33%) tumors without local recurrence (P=0.1850).
Discussion
According to previous expression array studies,28, 29 synovial sarcomas express the oncogene MYCN. Significant levels of expression of MYCN were not found in other sarcomas, including liposarcoma, clear cell sarcoma and fibrosarcoma, and MYCN thus served as one of the genes differentiating synovial sarcoma from other spindle cell tumors.28 In the current study, the majority of synovial sarcomas expressed MYCN by RT-PCR and almost 50% expressed MYCN at high levels. No correlation between histological subtype and MYCN expression was found, which is in keeping with the findings of Nagayama et al.29 Of the other sarcomas, a minority of malignant peripheral nerve sheath tumors and Ewing sarcoma/primitive neuroectodermal tumors expressed MYCN at low levels only; none of the non-synovial sarcomas expressed MYCN at high levels. The low levels of MYCN expression seen in malignant peripheral nerve sheath tumors is in keeping with a previous expression array study, where malignant peripheral nerve sheath tumors were found to express MYCN at levels lower than that seen in synovial sarcomas.29 Thus, in the current series of pediatric sarcomas, only synovial sarcomas expressed MYCN at high levels and was a useful test for distinguishing synovial sarcomas from other sarcomas with a specificity of 100% but sensitivity of only 42%, as several cases of synovial sarcoma expressed MYCN at low levels or not at all.
None of the cases of synovial sarcoma showed MYCN gene amplification. The lack of genomic amplification of MYCN in synovial sarcoma suggests alternative mechanisms are responsible for the high MYCN expression, such as alterations in transcriptional activity37 or dysregulation of protein degradative pathways, as described in neuroblastoma.38, 39 In neuroblastoma, the clinical significance of high levels of MYCN expression without gene amplification remains controversial; some studies suggest no clinical significance,40, 41 whereas others have reported a significant association with poorer outcome in a subset of older patients but not in infants.42 In the present series of synovial sarcoma, a high level of MYCN expression in the absence of MYCN amplification was not associated with a significant difference in local recurrence, metastatic rate or overall survival.
The expression of several downstream targets of MYCN in synovial sarcoma was also assessed. MRP1 encodes a member of the superfamily of ATP-binding cassette transporters, and functions as a multispecific organic anion transporter.43 Increased expression of MRP1 is associated with increased drug resistance and enhanced MRP1-mediated drug efflux.18 MCM7 is a DNA-binding protein essential for replication of DNA during the transition of G1 to S phase of the cell cycle,44 and increased expression of MCM7 has been demonstrated in proliferating tissues.45 ID2 is a helix-loop-helix transcription factor46 that binds to and inactivates the RB protein, thus stimulating cell proliferation by inhibiting the RB tumor suppressor pathway.20 Nestin is an intermediate filament and is thought to play a role in tumor development and aggressiveness.47, 48 MYCN has been shown to bind directly to the promoters of all four genes,18, 19, 20, 21 resulting in increased levels of expression of the resultant proteins in MYCN-amplified tumors. Although the current study did not find a significant correlation between high levels of MYCN expression and expression of downstream targets MRP1, MCM7 and nestin, there was a trend toward significance with expression of ID2. The lack of statistical significance raises two possible explanations. Firstly, the number of tumors in the present series is relatively small, and a larger series may be required to show a statistically significant correlation. For example, ID2 was expressed in 67% of synovial sarcomas with low or high levels of MYCN expression, but was not expressed at all in synovial sarcomas negative for MYCN. Secondly, the expression of such targets may be controlled by factors other than MYCN. For example, the MCM7 protein has numerous transactivation sites for the E2F transcription factor,49 and expression of MCM7 is increased by activation of E2F in fibroblasts.50 Furthermore, the nestin gene has several putative binding sites for the transcription factors HIF-2α and GATA within its first intron,51 suggesting a role for multiple transcription factors in nestin expression.
The present study did not show a significant correlation between expression of MYCN or downstream targets with progression-free survival, overall survival or metastases at presentation. Nonetheless, all cases that recurred locally were ID2-positive, compared with only three of nine cases that did not recur. This result, although not statistically significant in our small series, is worthy of further investigation. Overexpression of ID2 results in transformation of NIH 3T3 fibroblasts in vitro,52 and embryonic fibroblasts from ID2-null mice show noticeably lower rates of division. Furthermore, the correlation of MYCN and ID2 expression has been described in neuroblastoma,52 and it has been suggested that expression of ID2 is a better indicator of poor outcome than MYCN amplification. Such results provide a possible explanation for the oncogenic mechanism of MYCN overexpression in vivo.52 However, the prognostic significance of ID2 expression has been refuted by others, with some reporting no such association between ID2 expression and poor outcome in neuroblastomas and neuroblastoma cell lines.53
The current study identified a group of synovial sarcomas with high levels of MYCN expression. Such overexpression was not seen in other sarcomas entering the differential diagnosis of synovial sarcoma, and provides evidence that testing for MYCN expression may be a useful ancillary investigation in the differentiation of pediatric-spindled and monomorphic sarcomas. The biological and prognostic significance of such a finding is as yet unclear and deserves further study.
References
Coffin CM, Dehner LP, O'Shea PA . Pediatric Soft Tissue Sarcomas: A Clinical, Pathological and Therapeutic Approach. Williams and Wilkins: Baltimore, 1997.
Fisher C, de Bruijn DRH, van Kessel AG . Synovial sarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds). World Health Organization Classification of Tumours: Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 200–204.
Schmidt D, Thum P, Harms D, et al. Synovial sarcoma in children and adolescents. A report from the Kiel Pediatric Tumor Registry. Cancer 1991;67:1667–1672.
Weiss SW, Goldblum JR . Enzinger and Weiss's Soft Tissue Tumors, 4th edn. Mosby: St Louis, 2001, pp 1483–1509.
Turc-Carel C, Dal Cin P, Limon J, et al. Involvement of chromosome X in primary cytogenetic change in human neoplasia: nonrandom translocation in synovial sarcoma. Proc Natl Acad Sci USA 1987;84:1981–1985.
de Leeuw B, Berger W, Sinke RJ, et al. Identification of a yeast artificial chromosome (YAC) spanning the synovial sarcoma-specific t(X;18)(p11.2;q11.2) breakpoint. Genes Chromosomes Cancer 1993;6:182–189.
Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet 1994;7:502–508.
Crew AJ, Clark J, Fisher C, et al. Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J 1995;14:2333–2340.
dos Santos NR, de Bruijn DR, van Kessel AG . Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosomes Cancer 2001;30:1–14.
Skytting B, Nilsson G, Brodin B, et al. A novel fusion gene, SYT-SSX4, in synovial sarcoma. J Natl Cancer Inst 1999;91:974–975.
Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med 1998;338:153–160.
Somers GR, Gupta AA, Doria AS, et al. Pediatric undifferentiated sarcoma of the soft tissues: a clinicopathologic study. Pediatr Dev Pathol 2006;9:132–142.
Kempson RL, Fletcher CDM, Evans HL, et al. Tumors of the Soft Tissues, Vol. 30, 3rd edn. Armed Forces Institute of Pathology: Washington, DC, 2001.
Guillou L, Coindre J, Gallagher G, et al. Detection of the synovial sarcoma translocation t(X;18) (SYT;SSX) in paraffin-embedded tissues using reverse transcriptase-polymerase chain reaction: a reliable and powerful diagnostic tool for pathologists. A molecular analysis of 221 mesenchymal tumors fixed in different fixatives. Hum Pathol 2001;32:105–112.
Smith TA, Machen SK, Fisher C, et al. Usefulness of cytokeratin subsets for distinguishing monophasic synovial sarcoma from malignant peripheral nerve sheath tumor. Am J Clin Pathol 1999;112:641–648.
O'Sullivan MJ, Kyriakos M, Zhu X, et al. Malignant peripheral nerve sheath tumors with t(X;18). A pathologic and molecular genetic study. Mod Pathol 2000;13:1336–1346.
Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med 1985;313:1111–1116.
Manohar CF, Bray JA, Salwen HR, et al. MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma. Oncogene 2004;23:753–762.
Shohet JM, Hicks MJ, Plon SE, et al. Minichromosome maintenance protein MCM7 is a direct target of the MYCN transcription factor in neuroblastoma. Cancer Res 2002;62:1123–1128.
Lasorella A, Noseda M, Beyna M, et al. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 2000;407:592–598.
Thomas SK, Messam CA, Spengler BA, et al. Nestin is a potential mediator of malignancy in human neuroblastoma cells. J Biol Chem 2004;279:27994–27999.
Driman D, Thorner PS, Greenberg ML, et al. MYCN gene amplification in rhabdomyosarcoma. Cancer 1994;73:2231–2237.
Garson JA, McIntyre PG, Kemshead JT . N-myc amplification in malignant astrocytoma. Lancet 1985;2:718–719.
Garson JA, Pemberton LF, Sheppard PW, et al. N-myc gene expression and oncoprotein characterisation in medulloblastoma. Br J Cancer 1989;59:889–894.
Squire J, Goddard AD, Canton M, et al. Tumour induction by the retinoblastoma mutation is independent of N-myc expression. Nature 1986;322:555–557.
Nisen PD, Zimmerman KA, Cotter SV, et al. Enhanced expression of the N-myc gene in Wilms' tumors. Cancer Res 1986;46:6217–6222.
Mizukami Y, Nonomura A, Takizawa T, et al. N-myc protein expression in human breast carcinoma: prognostic implications. Anticancer Res 1995;15:2899–2905.
Segal NH, Pavlidis P, Antonescu CR, et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol 2003;163:691–700.
Nagayama S, Katagiri T, Tsunoda T, et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res 2002;62:5859–5866.
Karube K, Nabeshima K, Ishiguro M, et al. cDNA microarray analysis of cancer associated gene expression profiles in malignant peripheral nerve sheath tumours. J Clin Pathol 2006;59:160–165.
Miller SJ, Rangwala F, Williams J, et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res 2006;66:2584–2591.
Staege MS, Hutter C, Neumann I, et al. DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res 2004;64:8213–8221.
Prieur A, Tirode F, Cohen P, et al. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 2004;24:7275–7283.
Fligman I, Lonardo F, Jhanwar SC, et al. Molecular diagnosis of synovial sarcoma and characterization of a variant SYT-SSX2 fusion transcript. Am J Pathol 1995;147:1592–1599.
Boerner S, Squire J, Thorner P, et al. Assessment of MYCN amplification in neuroblastoma biopsies by differential polymerase chain reaction. Pediatr Pathol 1994;14:823–832.
Thorner PS, Ho M, Chilton-MacNeill S, et al. Use of chromogenic in situ hybridization to identify MYCN gene copy number in neuroblastoma using routine tissue sections. Am J Surg Pathol 2006;30:635–642.
Sivak LE, Tai KF, Smith RS, et al. Autoregulation of the human N-myc oncogene is disrupted in amplified but not single-copy neuroblastoma cell lines. Oncogene 1997;15:1937–1946.
Wada RK, Seeger RC, Brodeur GM, et al. Human neuroblastoma cell lines that express N-myc without gene amplification. Cancer 1993;72:3346–3354.
Cohn SL, Salwen H, Quasney MW, et al. Prolonged N-myc protein half-life in a neuroblastoma cell line lacking N-myc amplification. Oncogene 1990;5:1821–1827.
Nisen PD, Waber PG, Rich MA, et al. N-myc oncogene RNA expression in neuroblastoma. J Natl Cancer Inst 1988;80:1633–1637.
Slavc I, Ellenbogen R, Jung WH, et al. myc gene amplification and expression in primary human neuroblastoma. Cancer Res 1990;50:1459–1463.
Bordow SB, Norris MD, Haber PS, et al. Prognostic significance of MYCN oncogene expression in childhood neuroblastoma. J Clin Oncol 1998;16:3286–3294.
Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992;258:1650–1654.
Maiorano D, Lemaitre JM, Mechali M . Stepwise regulated chromatin assembly of MCM2-7 proteins. J Biol Chem 2000;275:8426–8431.
Todorov IT, Werness BA, Wang HQ, et al. HsMCM2/BM28: a novel proliferation marker for human tumors and normal tissues. Lab Invest 1998;78:73–78.
Norton JD . ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci 2000;113:3897–3905.
Weggen S, Bayer TA, Koch A, et al. Characterization of neural cell lines derived from SV40 large T-induced primitive neuroectodermal tumors. Brain Pathol 1997;7:731–739.
Dahlstrand J, Collins VP, Lendahl U . Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 1992;52:5334–5341.
Suzuki S, Adachi A, Hiraiwa A, et al. Cloning and characterization of human MCM7 promoter. Gene 1998;216:85–91.
Ohtani K, Iwanaga R, Nakamura M, et al. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene 1999;18:2299–2309.
Aihara M, Sugawara K, Torii S, et al. Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Invest 2004;84:1581–1592.
Lasorella A, Boldrini R, Dominici C, et al. Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res 2002;62:301–306.
Vandesompele J, Edsjo A, De Preter K, et al. ID2 expression in neuroblastoma does not correlate to MYCN levels and lacks prognostic value. Oncogene 2003;22:456–460.
Acknowledgements
We thank Dr Peizhu Sun and Ms Nicole Fabricius for technical assistance and Dr Cynthia Hawkins for help with the statistical analyses. Gratitude is extended to Dr Alberto Pappo for many helpful discussions regarding the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Somers, G., Zielenska, M., Abdullah, S. et al. Expression of MYCN in pediatric synovial sarcoma. Mod Pathol 20, 734–741 (2007). https://doi.org/10.1038/modpathol.3800792
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800792
This article is cited by
-
Etiopathogenic role of ERK5 signaling in sarcoma: prognostic and therapeutic implications
Experimental & Molecular Medicine (2023)
-
Nestin expression in osteosarcomas and derivation of nestin/CD133 positive osteosarcoma cell lines
BMC Cancer (2008)