Abstract
A wide variety of pulmonary and pleural histological changes is recognized in the setting of spontaneous pneumothorax. In this study, we describe a previously unreported lesion that was encountered in four males, 24–41 years of age. In addition to reactive eosinophilic pleuritis, subpleural emphysematous blebs, prominent eosinophilic exudate and lung atelectasis, the histology comprised exuberant type 2 pneumocyte hyperplasia, which was atypical enough to consider a diagnosis of adenocarcinoma in all four cases. Lung atelectasis and localized acute lung injury are factors likely responsible for this unusual histology, and along with the clinical history are important in recognizing the benign nature of this lesion. Awareness of this severe pneumocyte reaction in the setting of pneumothorax can help to prevent misdiagnosis as malignancy.
Similar content being viewed by others
Main
The histological changes following spontaneous pneumothorax include a wide spectrum of nonspecific findings, including emphysema with cyst formation, fibrosis, chronic inflammation, pigment deposition, bronchiolar, alveolar and mesothelial cell proliferation, vascular changes and reactive eosinophilic pleuritis.1, 2, 3 Unusual lesions, such as vasculopathy with or without eosinophils, are also described.4, 5 If taken out of context, these lesions can be diagnostically difficult and misleading. Here, we report another unusual manifestation of spontaneous pneumothorax characterized by prominent type 2 pneumocyte hyperplasia. To the best of our knowledge such prominent pneumocyte hyperplasia, in the setting of spontaneous pneumothorax, is not previously documented in the literature.
Materials and methods
The case material for this study came from the files of the Armed Forces Institute of Pathology and consultation files of one of the co-authors (TVC). The cases were accrued over the period from February 2003 to July 2004. Two to 11 H&E-stained slides (mean, 5) were available for review. Immunohistochemical studies with TTF-1 (8G7G3/1, pre-dilute solution) and CD68 (KP-1, 1:3000) were performed utilizing commercially available antibodies from Dako, Carpinteria, CA, USA. Clinical and follow-up information was obtained from the charts and from the contributing pathologists.
Results
Four male patients, mean age 31 (range, 24–41 years) comprised the study group, Table 1. They presented with symptoms typical of pneumothorax, including shortness of breath and pleuritic chest pain. Two of three patients were smokers. One patient had a history of hypercholesterolemia and hypertension. Two of four patients had recurrent pneumothorax prior to the tissue biopsy. No mass lesions were reported during the surgical procedure or on gross examination of the specimen. Based on the histology (see below), the possibility of malignancy was considered in all four cases. One patient was lost to follow-up, the other three were alive and well without any sign of a lung neoplasm at 30, 42, and 43 months of follow-up.
Histological Findings
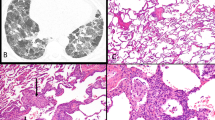
At scanning light microscopic examination, the cases showed increased cellularity (Figure 1A) that was in part due to atelectasis. In these areas, there was an exuberant type 2 pneumocyte hyperplasia with increased mitotic activity simulating the solid growth pattern of adenocarcinoma (Figure 1B). Cytologically, type 2 pneumocytes comprised cuboidal to columnar cells with prominent nuclei and vesicular chromatin. Occasional atypical mitoses were present. In better inflated regions, type 2 pneumocytes proliferated along the alveolar walls mimicking the lepidic growth of bronchioloalveolar carcinoma (Figure 1C, D). There was no nuclear overlapping, nuclear hyperchromasia or prominent nuclear membrane irregularities. These findings were observed in the background of reactive changes associated with eosinophilic pleuritis including fibrous pleural thickening with eosinophils, exuberant fibrinous exudate, pulmonary edema and prominent tissue eosinophilia (Figure 1E and F); in fact, some fields (two of four cases) resembled eosinophilic pneumonia. Other changes included fibrous pleural adhesions (two of four cases), subpleural emphysematous blebs, emphysema, respiratory bronchiolitis (four of four cases), smooth muscle metaplasia and focal medial and intimal thickening of pulmonary arteries (one of four cases). Type 2 pneumocyte hyperplasia, in all cases, was accompanied by a variable amount of intraalveolar organizing fibrin, Masson bodies and interstitial fibromyxoid change (Figure 1G). Type 2 pneumocytes were highlighted on immunohistochemical studies with TTF-1 (Figure 1H), while a majority of the intraalveolar cells were immunoreactive with CD68, confirming the presence of alveolar macrophages.
Atypia in pneumothorax. Areas of increased cellularity and parenchymal collapse are present in the center of the field (A), case 3, × 20. At higher magnification, they show densely packed type 2 pneumocytes with frequent mitotic figures resembling the solid growth pattern of adenocarcinoma (B), × 400. Prominent type 2 pneumocyte hyperplasia simulates the lepidic growth pattern, finding that is a feature of bronchioloalveolar carcinoma (C, D), × 200 and × 400, respectively. However, note the innocuous cytological features (B, D) comprising open nuclear chromatin and overall nuclear uniformity as well as the absence of nuclear polarization, hyperchromasia and contour irregularities. Additionally, the background changes are those of a typical eosinophilic pleuritis. There is exuberant fibrinous exudate overlying thickened pleura (E), × 100. Underlying lung displays conspicuous eosinophils admixed with alveolar macrophages (F), × 200. There are areas showing intraalveolar organizing fibrin, accompanied by interstitial fibromyxoid change and pneumocyte hyperplasia (G), the findings typically observed in organizing phase of diffuse alveolar damage, case 2, × 200. Hyperplastic type 2 pneumocytes of the lesion are immunoreactive with TTF-1 (H), × 200.
Discussion
Spontaneous pneumothorax is known to elicit a range of reactive epithelial changes including mesothelial hyperplasia, columnar (Dunnill lesion) or mucus cell metaplasia of the pleura and type 2 pneumocyte hyperplasia in the underlying lung.1, 6, 7 Chronic pneumothorax can lead to squamous metaplasia of the pleural surface.8 Emphysema with cyst formation (so-called ‘blebs’), atelectasis, fibrosis, chronic inflammation, fluid accumulation, eosinophilic infiltrates and vasculopathy are other tissue reactions (some probably preexisting) documented in the setting of pneumothorax.1, 2, 3, 4, 5, 9, 10, 11, 12, 13
The four cases presented herein exemplify a previously unrecognized manifestation of spontaneous pneumothorax showing exuberant type 2 pneumocyte hyperplasia. Focally, this proliferation was prominent enough to include adenocarcinoma in the differential diagnosis. However, several clinical findings point to the benign nature of this lesion: the relatively young age of the patients, the history of pneumothorax, and the absence of mass lesions radiologically, during the surgical procedure and on gross examination. Furthermore, the observed epithelial changes were associated with localized acute lung injury (ALI), and cytological atypia is well recognized in that setting. While rarely eosinophilic infiltrates can occur in primary lung carcinomas, the finding of an eosinophilic pneumonia type reaction, as seen in our cases, would also be unusual in a carcinoma. In addition, several cytomorphological features, including the preservation of nuclear cytoplasmic ratio with open nuclear chromatin, the absence of nuclear stratification, nuclear hyperchromasia and nuclear membrane irregularities, support the benign nature of this process. It is not certain which factors are responsible for this unique histology. While atelectasis is a common feature in pneumothorax, the localized ALI with exuberant pneumocyte hyperplasia is unique to this set of cases, and perhaps, is the factor making these cases histologically distinct.
Type 2 pneumocyte hyperplasia is a universal reaction in injured lung.3, 14 It is most striking in diffuse alveolar damage (DAD), but is also seen in organizing pneumonia, non-specific interstitial pneumonia, and in a variety of other settings, including acute bronchopneumonia, in the lung surrounding granulomas, lesions of pulmonary Langerhans’ cell histiocytosis, tumors and abscesses. Pneumocyte hyperplasia is commonly observed in the lung adjacent to pleuritis but on a practical basis is considered a minor finding that is rarely reported. In their original paper on the subject of spontaneous pneumothorax, Lichter and Gwynne documented pneumocyte hyperplasia in 11 of 20 (55%) cases.1 However, according to the illustrations and microscopic description, this phenomenon was of a much lesser degree than that observed in our cases and was considered to be related to nearby scarring and chronic inflammation. The extent of type 2 pneumocyte proliferation in our cases exceeds that typically seen in pneumothorax, and it is not certain which host or exogenous factors are responsible for this phenomenon and we are not certain whether it is a consequence of or a contributing cause of pneumothorax.
Recognition of the reactive nature of type 2 pneumocyte hyperplasia in DAD in histologic material is not difficult. Preservation of the nuclear cytoplasmic ratio in combination with the exudative or fibromyxoid milieu of DAD helps to arrive at the correct diagnosis.3 However, the separation of hyperplastic type 2 pneumocytes from adenocarcinoma is more problematic in cytological preparations, where architectural evaluation is not possible, and clinical findings are considered to be more helpful in ruling out a diagnosis of malignancy.15, 16 Similar to cytological preparations, the atelectasis that is a common consequence of pneumothorax, precludes adequate evaluation of lung architecture and therefore can hamper the evaluation of epithelial changes.
As the underlying lung injury in our cases is an incidental localized microscopic finding, its exact classification is somewhat problematic. However, in our view, it may be akin to what was previously reported as regional DAD by Yazdy et al.17 While no reason for localization of DAD within the lung was clearly identified in that study, it was speculated that the regional DAD could result from irregular blood flow related to vascular occlusion, irregularly distributed microvascular injury, or from a combination of factors.17 The epithelial changes in ALI that mimic epithelial malignancy are not restricted to type 2 pneumocytes. Exuberant squamous metaplasia resembling the histology of squamous cell carcinoma in the setting of DAD has been documented previously by Ogino et al.18 From our experience and reports in the literature,19, 20, 21, 22, 23 other common reactive processes in the lung that can mimic carcinoma include peribronchiolar metaplasia, type 2 pneumocyte hyperplasia or squamous metaplasia around bronchioles in the setting of post-radiation therapy or chemotherapy, exuberant peribronchiolar metaplasia (Lambertosis) associated with bronchiolitis of any cause, and squamous metaplasia in tracheobronchial ulceration, within cavitary abscesses, around infarcts and around fibrous scars.
In summary, prominent type 2 pneumocyte hyperplasia can occur in the settings of spontaneous pneumothorax and, similarly to other reactive processes such as squamous metaplasia, can mimic epithelial malignancy in the lung. Attention to clinical presentation and background histological changes in the pleura and lung parenchyma is critical to the recognition of the benign nature of this lesion.
References
Lichter I, Gwynne JF . Spontaneous pneumothorax in young subjects. A clinical and pathological study. Thorax 1971;26:409–417.
Askin FB, McCann BG, Kuhn C . Reactive eosinophilic pleuritis: a lesion to be distinguished from pulmonary eosinophilic granuloma. Arch Pathol Lab Med 1977;101:187–191.
Travis WD, Colby TV, Koss MN, et al. Non-neoplastic disorders of the lower respiratory tract. In: King DW (ed). Atlas of Nontumor Pathology. American Registry of Pathology and Armed Forces Institute of Pathology: Washington, DC, 2002.
Luna E, Tomashefski Jr JF, Brown D, et al. Reactive eosinophilic pulmonary vascular infiltration in patients with spontaneous pneumothorax. Am J Surg Pathol 1994;18:195–199.
Cyr PV, Vincic L, Kay JM . Pulmonary vasculopathy in idiopathic spontaneous pneumothorax in young subjects. Arch Pathol Lab Med 2000;124:717–720.
Bashir MS, Cowen PN . Mucous metaplasia of the pleura. J Clin Pathol 1992;45:1030–1031.
Dunhill MS . Metaplastic changes in the visceral pleura in a case of fibrocystic disease of the pancreas. J Pathol Bacteriol 1959;77:299–302.
Willen R, Bruce T, Dahlstrom G, et al. Squamous epithelial cancer in metaplastic pleura following extrapleural pneumothorax for pulmonary tuberculosis. Virchows Arch A Pathol Anat Histol 1976;370:225–231.
Cottin V, Streichenberger N, Gamondes JP, et al. Respiratory bronchiolitis in smokers with spontaneous pneumothorax. Eur Respir J 1998;12:702–704.
Kay JM . Pulmonary vasculopathy and recurrent pneumothoraces. Chest 1997;111:1784–1785.
McDonnell TJ, Crouch EC, Gonzalez JG . Reactive eosinophilic pleuritis. A sequela of pneumothorax in pulmonary eosinophilic granuloma. Am J Clin Pathol 1989;91:107–111.
Schnader J, Terry PB, Field SK, et al. Clinical conference on management dilemmas. Pulmonary vasculopathy and recurrent pneumothoraces. Chest 1996;110:1340–1347.
Tomashefski Jr JF, Dahms B, Bruce M . Pleura in pneumothorax. Comparison of patients with cystic fibrosis and idiopathic spontaneous pneumothorax. Arch Pathol Lab Med 1985;109:910–916.
Castranova V, Rabovsky J, Tucker JH, et al. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol 1988;93:472–483.
Grotte D, Stanley MW, Swanson PE, et al. Reactive type II pneumocytes in bronchoalveolar lavage fluid from adult respiratory distress syndrome can be mistaken for cells of adenocarcinoma. Diagn Cytopathol 1990;6:317–322.
Stanley MW, Henry-Stanley MJ, Gajl-Peczalska KJ, et al. Hyperplasia of type II pneumocytes in acute lung injury. Cytologic findings of sequential bronchoalveolar lavage. Am J Clin Pathol 1992;97:669–677.
Yazdy AM, Tomashefski Jr JF, Yagan R, et al. Regional alveolar damage (RAD). A localized counterpart of diffuse alveolar damage. Am J Clin Pathol 1989;92:10–15.
Ogino S, Franks TJ, Yong M, et al. Extensive squamous metaplasia with cytologic atypia in diffuse alveolar damage mimicking squamous cell carcinoma: a report of 2 cases. Hum Pathol 2002;33:1052–1054.
Fukuda Y, Ishizaki M, Masuda Y, et al. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol 1987;126:171–182.
Fulkerson WJ, McLendon RE, Prosnitz LR . Adult respiratory distress syndrome after limited thoracic radiotherapy. Cancer 1986;57:1941–1946.
Corrin B . Pathology of the lungs, 2nd edn. Elsevier Churchill Livingstone: Edinburgh, 2005.
Couture C, Colby TV . Histopathology of bronchiolar disorders. Semin Respir Crit Care Med 2003;24:489–498.
Colby TV . Bronchiolitis. Pathologic considerations. Am J Clin Pathol 1998;109:101–109.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions and assertions contained herein are the express views of the authors and are not to be construed as official or reflecting the views of the Departments of the Army or Defense. This is a US Government work, and as such, is in the public domain in the United States of America.
Rights and permissions
About this article
Cite this article
Shilo, K., Colby, T., Travis, W. et al. Exuberant type 2 pneumocyte hyperplasia associated with spontaneous pneumothorax: secondary reactive change mimicking adenocarcinoma. Mod Pathol 20, 352–356 (2007). https://doi.org/10.1038/modpathol.3800744
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800744