Abstract
The relationship between HER-2 overexpression and gene amplification is well evaluated in breast cancers but remains unclear or controversial in many other tumor entities. Therefore, we tested the HER-2 status in more than 120 different tumor entities. 5751 tumor samples were analyzed on TMAs by immunohistochemistry (Hercept-Test, DAKO) and fluorescence in situ hybridization (PathVysion, Abbott-Vysis) under highly standardized conditions. HER-2 overexpression (score 2/3+) and amplification occurred most often in breast cancers but was also seen in 18 other tumor entities including cancers of the urinary bladder (amplification in 14.3%, overexpression in 6.7%), stomach (8.3/4.9%), endometrium (6.6/6.8%), lung (2.8/3.1%) and ovary (2.3/1.2%). Remarkably, a strong association between overexpression and amplification was seen in all examined cancer entities. Trastuzumab therapy is highly efficient in HER-2 amplified breast cancer both in metastatic disease and as an adjuvant therapy. A variety of other tumor entities including frequent neoplasms and cancers with often limited therapeutic options have similar patterns of HER-2 alterations as observed in breast cancer (ie high overexpression due to high level gene amplification). Such tumor entities should be carefully evaluated for a possible utility of trastuzumab treatment.
Similar content being viewed by others
Main
The proto-oncogene HER-2 is involved in the development of numerous types of human cancer and has been intensely evaluated as therapeutic target.1, 2, 3 HER-2 gene amplification and protein overexpression occurs in about 20% of breast cancers4 and is linked to poor prognosis in these tumors.5 More importantly, HER-2 is the target of an antibody based therapy (trastuzumab) which is routinely used in metastatic HER-2 positive breast cancer.6, 7, 8 More recently, adjuvant trastuzumab application was shown to be dramatically effective in HER-2 positive breast cancer patients, too.9
The potential benefit of trastuzumab in other tumor entities is largely unknown. HER-2 positivity has been described in most human tumor types but with a highly variable frequency. This especially applies for immunohistochemistry (IHC) analyses where the use of different reagents and definitions resulted in an extremely wide range of HER-2 positivity. For example, HER-2 overexpression was shown in 5.7–88.8% of non-small-cell lung cancers10, 11 and 3.0–54% of colon cancers.12, 13 To a smaller extent, this variability is also observed in amplification analysis. Different methods for analysis (Southern blot or fluorescent in situ hybridization (FISH)) and definitions of amplification have resulted in variable frequencies of amplification reported in the literature such as 0–66% in ovarian cancer14, 15 or 6–56.2% in breast cancer.15, 16
In this study, HER-2 overexpression and HER-2 amplification were analyzed in more than 3000 tumors from >120 different tumor categories using FDA (US Food and Drug Administration) approved methods for immunohistochemistry (HercepTest, DAKO) and fluorescent in situ hybridization (PathVysion, Abbott-Vysis). In order to obtain most comparable data, tissue microarrays (TMA) were utilized.17 In this method, thousands of tissues can be analyzed on a few slides on one day in one set of reagents thus allowing maximal standardization of analysis. This study design allowed comparison of HER-2 gene amplification and HER-2 protein overexpression in many different human tumors. The fraction of highly amplified cases among HER-2 overexpressing cancers is of particular interest as breast cancer studies suggested that amplified cancers might benefit most from trastuzumab therapy.18
Materials and methods
Tissue Microarrays
Two sets of pre-existing tissue microarrays (TMAs) were used for this study. The first set consisted of 2197 breast cancers,19 the second included 1–50 samples of more than 125 different tumor types and subtypes (total: 3554 tumors). The exact composition of these TMAs is described in the results section (Tables 1 and 2). All tissues were formalin-fixed and paraffin-embedded. TMAs were constructed as previously described.17 In brief, tissue cylinders were punched from representative tumor areas of the donor paraffin block. Consecutively, the tissue sample was placed in the recipient paraffin block using a home made semiautomatic precision instrument. One TMA contained up to a maximum of 612 tumors tissue spots with a diameter of 0.6 mm each. Four micrometers TMA sections were prepared using an adhesive coated slide system (Instrumedics).
Immunohistochemistry
The HercepTest (DAKO, Glostrup, Denmark) was used according to the protocol of the manufacturer. Antigen retrieval of the deparaffinized tissue sections was performed in a waterbath at 95–99°C for 50 min followed by peroxidase blocking and incubation with the pre-diluted primary antibody. Cell line test slides provided by the manufacturer were used as positive and negative controls. Immunostaining was scored by one pathologist (CT) according to the manufacturer's directions.
Fluorescent In Situ Hybridization
For proteolytic slide pre-treatment a commercial kit was utilized (Paraffin pre-treatment reagent kit, Vysis, Downers Grove, IL, USA). Spectrum-Orange-labelled HER-2 probes were used together with Spectrum-Green-labelled centromere 17 reference probes (PathVysion™ Vysis). Before hybridization, sections were deparaffinized, air dried, dehydrated and then denaturated for 5 min at 74°C in 70% formamide-2 × SSC solution. After overnight hybridization at 37°C in a humid chamber, slides were washed and counterstained with 0.2 μM DAPI in an antifade solution. A tumor was considered amplified if the estimated ratio of HER-2/centromere 17 was ≥2.0.
Results
Immunohistochemistry
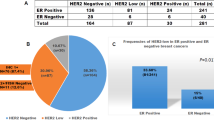
IHC was interpretable in 4467 of 5751 tumor spots (77.7%). Reasons for non-informative results were either missing tissue spots or absence of tumor tissue. HER-2 overexpression was most frequently seen in breast cancers, where a 3+ result was seen in 216 (10.2%) and 2+ positivity in 64 (3.0%) of 2114 tumors. HER-2 positivity (2/3+) was also observed in 11 other tumor types and subtypes (Table 1). Among these tumors, positive cases were particularly frequent in endometrium (6.8%), stomach (4.9%) and invasive urothelial cancers (6.7%). IHC results were negative (0/1+) in 120 other tumor categories (Tables 1 and 2).
Fluorescence In Situ Hybrdization
3984 of 5751 (69.3%). tumor spots could be analyzed by FISH. Reasons to exclude cases were either missing tissue spots or absence of tumor tissue as in IHC. In addition, there was a fraction of tissue spots with insufficient hybridization signals. As observed for IHC, the highest frequency of amplification was seen in breast cancers (16.9%). Amplification was observed in 18 additional tumor categories (Table 1). Among these, amplifications were most prevalent in invasive bladder cancer (14.3%), stomach cancer (8.3%), esophagus cancer (6.7%), pancreatic cancer (6.9%) and endometrial cancer (6.6%).
IHC and FISH
Both FISH and IHC were interpretable on the same tissue spot in 3211 of 5751 tumor samples (55.8%). There was a strong association between IHC positive cases and HER-2 amplifcation in breast cancers and an even better association in non-breast cancers (Figure 1). In tumors with an IHC score of 2+ a concomitant HER-2 amplification was observed in 60% non-breast cancers and 48.9% breast cancers. Non-breast cancers with a score of 3+ were amplified in 100% of the cases while breast cancers were amplified in 91.4% only (Figure 1). In non-breast cancers and breast cancers without expression of HER-2 (score 0), HER-2 amplification was found in only 1.6% and in 4.7%, respectively.
Relationship between immunohistochemical overexpression and amplification of HER2 in breast and non-breast cancers. In both tumor groups the correlation becomes stronger with increasing levels of HER2 overexpression. The percentage of immunohistochemically positive tumors with coexistent amplification is slightly higher in non-breast cancers.
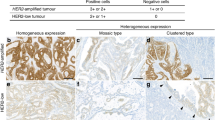
Absence of overexpression (score 0) despite of amplification was seen in six non-breast cancer specimens. Two of them revealed a borderline FISH result (ratio≤3.0; maximal HER-2 gene copy number 10) which may have resulted in a low level of expression not detectable by IHC.20 Four cases with a high level (>3.0) HER-2 amplification but negative IHC result (score 0) are most likely explained by false negative IHC. Examples of tumors with HER-2 overexpression and amplification are shown in Figure 2.
Discussion
The HER-2 gene product is a prime example of an extensively analyzed protein. More than 2000 studies have investigated HER-2 expression by IHC in breast cancer and more than 600 studies in other tumor entities. These studies have described a very wide range of HER-2 expression in many tumor types (reviewed in Sauter et al21). For example HER-2 positivity has been observed in 0–100% of renal and prostate cancers.22, 23 Such controversial data makes it evident that, for most cancer types, true HER-2 alteration frequencies can hardly be obtained from the literature. Therefore, we analyzed more than 3000 tumors of 120 different tumor subtypes under fully standardized conditions. This allowed us to give a reliable estimate of HER-2 overexpression/HER-2 amplification across most human tumor entities.
As expected, breast cancers were among the most frequently HER-2 positive tumors. This confirms the predominant importance of HER-2 for this cancer. HER-2 amplification was found in 16.9% of breast cancers, which is in line with published data.24 Our result of 13.2% HER-2 protein overexpression in breast cancer is in the lower range of published results for FDA approved reagents (13–30% positive).24, 25, 26 However, the strong correlation found between IHC and FISH in our breast cancer samples was exactly as described in the literature27, 28 (Figure 1). This confirms the validity of our assays.
Breast cancer is the only cancer type for which the rate of HER-2 positivity is currently well established (15–20%). Our ability to reproduce the expected breast cancer values in our TMA experiment provides indirect evidence that the frequencies of HER-2 positivity observed for other tumor entities also ranges close to the true HER-2 prevalence in these tumors. More than 15 additional tumor entities including clinically important cancers such as stomach, pancreatic, and bladder cancer also showed a relevant frequency of HER-2 amplification/overexpression. For all of these tumor entities, HER-2 positivity (overexpression and/or amplification) had been previously described.29, 30, 31, 32 For example, HER-2 positivity for gastric cancer has been reported to range between 8 and 56% (reviewed in Sauter et al21). Our data now suggest that the true prevalence of HER-2 amplification/overexpression may range between 6 and 10%. Comparable frequencies may also apply for esophagus, pancreas or bladder cancer.
Of most tumor categories, we analyzed around 50 cases or even fewer. This rather low number raises the possibility that rare HER-2 amplification/overexpression may have been missed in some tumor entities. A very low incidence of HER-2 amplifications may hence be present in a much higher number of cancer types than detected in our study. This assumption is corroborated by the example of colon cancer. While none of 41 colon cancers analyzed in this study was HER-2 positive, in a subsequent study analyzing 400 cancers on a colon cancer TMA, we found two (0.5%) highly amplified colon cancers (L Terracciano, unpublished data). That rare HER-2 positivity might have clinical utility for individual patients was demonstrated by a clinical trial of patients with salivary gland cancers. Of the 126 patients 14 with 2/3+ HER-2 positivity by IHC had been treated with trastuzumab.33 The trial was terminated early because the frequency of positivity was disappointingly low. However, bone metastases vanished in one patient under trastuzumab monotherapy suggesting strong response to therapy.
For breast cancer, recent data have strongly suggested, that only amplified cancers would respond to trastuzumab.18 Thorough breast cancer studies analyzing unfixed samples on the DNA, RNA and protein level have suggested a 1:1 relationship between protein overexpression and gene amplification.25 Interestingly, our data suggest a similar relationship between expression and amplification across all tumor types. It might therefore, be possible that gene amplification constitutes a universal predictor of trastuzumab response independent of the tumor type. The use of IHC for HER-2 testing may constitute one reason for disappointing results in early non-breast cancer trastuzumab trials.34 HER-2 IHC is prone to various technical problems including false positivity in case of inproper tissue fixation.35
The absence of HER-2 amplification and high level overexpression in some tumor types like prostate cancer36, 37 is also a noteworthy result of this study. Based on FISH studies using very low stringency for definition of HER-2 amplification and IHC studies using non-FDA approved reagents, prostate cancer has been suspected a potential target for trastuzumab.14 However, several carefully executed FISH and IHC studies have meanwhile been published and showed absence of HER-2 amplification and a very low frequency of HER-2 expression.38, 39 Also we found HER-2 positivity in <5% of our lung cancers. Earlier reports had described HER-2 positivity in up to 93%.40 The poor results of trastuzumab studies in lung cancer are an excellent example for risks involved in planning clinical trials based on published IHC results.
In summary, these data show that occasional HER-2 amplification can occur in many different tumor entities. As known for breast cancer, HER-2 overexpression appears to be rare in non-amplified tumors. It will be important to investigate whether highly amplified HER-2 positive non-breast cancers may benefit from trastuzumab therapy.
References
Hynes NE, Lane HA . ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341–354.
Normanno N, Bianco C, Strizzi L, et al. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets 2005;6:243–257.
Rabindran SK . Antitumor activity of HER-2 inhibitors. Cancer Lett 2005;227:9–23.
Zhang D, Salto-Tellez M, Do E, et al. Evaluation of HER-2/neu oncogene status in breast tumors on tissue microarrays. Hum Pathol 2003;34:362–368.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–182.
Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol 1999;26:78–83.
Leyland-Jones B . Trastuzumab: hopes and realities. Lancet Oncol 2002;3:137–144.
Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol 2004;22:1063–1070.
Tuma RS . Trastuzumab trials steal show at ASCO meeting. J Natl Cancer Inst 2005;97:870–871.
Ugocsai K, Mandoky L, Tiszlavicz L, et al. Investigation of HER2 overexpression in non-small cell lung cancer. Anticancer Res 2005;25:3061–3066.
Bakir K, Ucak R, Tuncozgur B, et al. Prognostic factors and c-erbB-2 expression in non-small-cell lung carcinoma (c-erbB-2 in non-small cell lung carcinoma). Thorac Cardiovasc Surg 2002;50:55–58.
Ooi A, Takehana T, Li X, et al. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: an immunohistochemical and fluorescent in situ hybridization study. Mod Pathol 2004;17:895–904.
Porebska I, Harlozinska A, Bojarowski T . Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol 2000;21:105–115.
Ross JS, Sheehan C, Hayner-Buchan AM, et al. HER-2/neu gene amplification status in prostate cancer by fluorescence in situ hybridization. Hum Pathol 1997;28:827–833.
Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 1999;5:1966–1975.
Ma Y, Lespagnard L, Durbecq V, et al. Polysomy 17 in HER-2/neu status elaboration in breast cancer: effect on daily practice. Clin Cancer Res 2005;11:4393–4399.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–847.
Mass RD, Press MF, Anderson S, et al. Evaluation of Clinical Outcomes According to HER2 Detection by Fluorescence In Situ Hybridization in Women with Metastatic Breast Cancer Treated with Trastuzumab. Clin Breast Cancer 2005;6:240–246.
Al-Kuraya K, Schraml P, Torhorst J, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res 2004;64:8534–8540.
Owens MA, Horten BC, Da Silva MM . HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004;5:63–69.
Sauter G, Simon R, Hillan K . Tissue microarrays in drug discovery. Nat Rev Drug Discov 2003;2:962–972.
Latif Z, Watters AD, Bartlett JM, et al. Gene amplification and overexpression of HER2 in renal cell carcinoma. BJU Int 2002;89:5–9.
Gu K, Mes-Masson AM, Gauthier J, et al. Overexpression of her-2/neu in human prostate cancer and benign hyperplasia. Cancer Lett 1996;99:185–189.
Sauer T, Wiedswang G, Boudjema G, et al. Assessment of HER-2/neu overexpression and/or gene amplification in breast carcinomas: should in situ hybridization be the method of choice? Apmis 2003;111:444–450.
Pauletti G, Dandekar S, Rong H, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 2000;18:3651–3664.
Bermont L, Algros MP, Baron MH, et al. Relevance of p185 HER-2/neu oncoprotein quantification in human primary breast carcinoma. Breast Cancer Res Treat 2000;63:163–169.
Couturier J, Vincent-Salomon A, Nicolas A, et al. Strong correlation between results of fluorescent in situ hybridization and immunohistochemistry for the assessment of the ERBB2 (HER-2/neu) gene status in breast carcinoma. Mod Pathol 2000;13:1238–1243.
Jacobs TW, Gown AM, Yaziji H, et al. Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol 1999;17:1974–1982.
Takehana T, Kunitomo K, Kono K, et al. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer 2002;98:833–837.
Ross JS, McKenna BJ . The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest 2001;19:554–568.
Hansel DE, Ashfaq R, Rahman A, et al. A subset of pancreatic adenocarcinomas demonstrates coamplification of topoisomerase IIalpha and HER2/neu: use of immunolabeling and multicolor FISH for potential patient screening andtreatment. Am J Clin Pathol 2005;123:28–35.
Latif Z, Watters AD, Dunn I, et al. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer 2004;40:56–63.
Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol 2003;39:724–727.
Clamon G, Herndon J, Kern J, et al. Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 2005;103:1670–1675.
Tapia C, Schraml P, Simon R, et al. HER2 analysis in breast cancer: reduced immunoreactivity in FISH non-informative cancer biopsies. Int J Oncol 2004;25:1551–1557.
Visakorpi T, Kallioniemi OP, Koivula T, et al. Expression of epidermal growth factor receptor and ERBB2 (HER-2/Neu) oncoprotein in prostatic carcinomas. Mod Pathol 1992;5:643–648.
Oxley JD, Winkler MH, Gillatt DA, et al. Her-2/neu oncogene amplification in clinically localised prostate cancer. J Clin Pathol 2002;55:118–120.
Bubendorf L, Kononen J, Koivisto P, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res 1999;59:803–806.
Lara Jr PN, Chee KG, Longmate J, et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer 2004;10:2125–2131.
Bongiorno PF, Whyte RI, Lesser EJ . Alterations of K-ras, p53, and erbB-2/neu in human lung adenocarcinomas. J Thorac Cardiovasc Surg 1994;107:590–595.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tapia, C., Glatz, K., Novotny, H. et al. Close association between HER-2 amplification and overexpression in human tumors of non-breast origin. Mod Pathol 20, 192–198 (2007). https://doi.org/10.1038/modpathol.3800729
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800729
Keywords
This article is cited by
-
Determination of Tumor Heterogeneity in Colorectal Cancers Using Heterogeneity Tissue Microarrays
Pathology & Oncology Research (2015)
-
HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions
Modern Pathology (2013)
-
TMA-Technik in der onkologischen Diagnostik
Forum (2012)
-
Assessment of ErbB2 (Her2) in oesophageal adenocarcinomas: summary of a revised immunohistochemical evaluation system, bright field double in situ hybridisation and fluorescence in situ hybridisation
Modern Pathology (2011)
-
Involvement of HER-2/neu and metastasis-related proteins in the development of ileal neuroendocrine tumors
Virchows Archiv (2011)