Abstract
Vulvar cancer represents an important medical problem worldwide whose incidence is increasing at an alarming rate in young females. Several factors have been linked to vulvar cancer development, but its exact pathogenesis remains to be determined. Vulvar tumorigenesis proceeds through intermediate dysplastic lesions, known as vulvar intraepithelial neoplasias, frequently associated with non-neoplastic epithelial disorders of the vulva, such as lichen sclerosus and squamous cell hyperplasia. In this study, the expression of the CDK inhibitor p27Kip1 and the extent of endogenous oxidative DNA damage were evaluated in vulvar specimens, including normal tissues, lichen sclerosus, squamous cell hyperplasia, vulvar intraepithelial neoplasias and invasive squamous cell carcinomas. We found that p27Kip1 was constantly expressed in normal vulvar epithelium cells while a progressive significant reduction in the percentage of p27Kip1-positive cells was observed in vulvar intraepithelial neoplasias (77%) and in invasive carcinomas (64%). Mean percentage of positive cells in invasive carcinomas, but not in vulvar intraepithelial neoplasias, was also significantly lower than squamous cell hyperplasia lesions (78%) while lichen sclerosus displayed a percentage of positive cells (45%) significantly lower than both vulvar intraepithelial neoplasias and invasive carcinomas. 8-hydroxydeoxyguanosine (8-OHdG) is considered a sensitive biomarker for oxidative stress. We observed a progressive significant increase in the levels of 8-OHdG and in the percentage of positive cells from normal vulvar epithelium to vulvar intraepithelial neoplasias (25%) and to invasive carcinomas (64%). Squamous cell hyperplasia displayed an intermediate percentage of positive cells comparable to vulvar intraepithelial neoplasias 2 but significantly higher than vulvar intraepithelial neoplasias 1 and lower than invasive carcinomas. Lichen sclerosus staining was significantly lower than carcinomas but higher than vulvar intraepithelial neoplasias and squamous cell hyperplasia. These results demonstrate that expression of p27Kip1 is downregulated while oxidative DNA damage increases from early non-neoplastic epithelial alterations through vulvar intraepithelial neoplasias to invasive vulvar carcinomas. Thus, both parameters might play an important role in the development of this cancer and their study might contribute to our understanding of human vulvar carcinogenesis.
Similar content being viewed by others
Main
Squamous cell carcinoma of the vulva accounts for 4% of all gynecologic malignancies and although its incidence rates in older women have remained relatively stable over the past few decades, diagnosed cases of vulvar cancer are increasing at an alarming rate in young females worldwide.1 The type of vulvar squamous cell carcinoma usually found in younger women appears to be associated with human papillomavirus (HPV) infection. Indeed, specific genital HPV types, in particular HPV 16, are strongly implicated in the causation of high-grade vulvar intraepithelial neoplasia (VIN), currently accepted as their precursor, and the incidence of HPV-related VIN is increasing worldwide. The most common form of the disease, however, mainly develops in women in their seventh and eighth decades of life and is frequently associated with non-neoplastic epithelial disorders of the vulva, such as lichen sclerosus, squamous cell hyperplasia and other dermatoses.2 Several conditions (ie, lymphogranuloma venereum, granuloma inguinale, immunosuppression, hypertension and other chronic dermatologic or metabolic diseases) have been associated with an increased risk of developing this disease and women with vulvar cancer have an increased risk for dysplasia and invasive carcinomas of cervix and vagina. In addition, tobacco use is also common in younger patients with vulvar cancer and exposure to tobacco smoke has been reported as an important risk factor for vulvar carcinogenesis.1 Chromosomal changes and angiogenesis have been reported to play a role in the process but the exact pathogenesis of vulvar squamous cell carcinoma remains still unclear and investigation on molecular markers involved in vulvar carcinogenesis is justified in order to clarify the natural history of this malignancy.
Deregulation of the normal cell cycle is a frequent event in human tumors and has been reported in vulvar tumorigenesis. Thus, an increased expression of p53 and Ki67 was reported to accompany histologic changes in the progression of vulvar premalignant lesions to malignancy.3 On the other hand, a progressive loss of pRb, pRb2/p130 and of the CDK inhibitor p16INK4 from benign lesions to invasive squamous cell carcinomas of the vulva has been reported4, 5, 6 thus suggesting a role for these molecules in vulvar carcinogenesis.
p27Kip1 is a negative regulator of the G1 phase of the cell cycle, is regarded as a tumor suppressor gene and is frequently lost in tumor cells.7 Loss of p27Kip1 expression has been reported in a subset of human vulvar squamous cell carcinomas but its role in human vulvar tumorigenesis remains unclear.6, 8
The reactive oxygen species formed during exposure to environmental oxidants and during endogenous metabolic processes, play an important role in the pathogenesis of cancer and other degenerative diseases.9 Reactive oxygen species can induce genotoxic damage, including single- and double-strand breaks, DNA–protein crosslinks, abasic sites and modified bases. Oxidation of the C8 of guanine is one of the most abundant types of oxidative DNA damage.10 The modified base 8-hydroxydeoxyguanosine (8-OHdG) is a mutagenic lesion in vivo and in vitro, leading to G-T and A-C substitutions.11 It is therefore suggested as a sensitive biomarker for molecular epidemiological assessment of cancer risk due to oxidative stress. An immunoperoxidase method for 8-OHdG detection in single cells has been developed using a specific anti-8-OHdG monoclonal antibody.12 This assay has shown sufficient sensitivity for detection of oxidative DNA damage in liver tissues from aflatoxin B1-treated rats and in a variety of human normal and cancer tissues from smokers and nonsmokers.13, 14 We previously optimized the method for the evaluation of oxidative DNA damage in human cells and have successfully applied this technique to detect 8-OHdG levels in human tissues.15, 16 Using this method to detect DNA oxidative damage, we demonstrated that 8-OHdG content is significantly higher in cervical cancer compared with normal cervical squamous epithelium cells and that an increase in oxidative DNA damage is associated with progression of cervical preinvasive lesions. We also demonstrated that expression of p27Kip1 is downregulated during cervical carcinogenesis and is inversely correlated with oxidative DNA damage.15, 17
Since the same risk factors (ie, cigarette smoke, HPV infection, sexual activity, etc) might be involved in the development of both cervical and vulvar cancers, in this study we investigated the role of these two markers in the less frequent vulvar carcinogenesis. The expression pattern of the p27kip1 protein and of 8-OHdG was analyzed by immunostaining in vulvar normal epithelium and in different vulvar lesions including lichen sclerosus, squamous cell hyperplasia, VIN and invasive squamous cell carcinomas.
Our results demonstrate that expression of p27Kip1 is downregulated while oxidative DNA damage increases during vulvar carcinogenesis. Both parameters are altered at early stages of the process and might play a role in the progression of vulvar neoplastic lesions. The implications of these findings are discussed.
Materials and methods
Samples
Samples were collected from patients who underwent surgery for vulvar lesions at the Policlinico ‘Agostino Gemelli’ in Rome, Italy. No patient received preoperative chemotherapy or radiotherapy.
Formalin-fixed, paraffin-embedded vulvar specimens were obtained from the files of the Department of Pathology. Surgical specimens included biopsies for diagnosis, wide excisions, hemivulvectomy and radical vulvectomy undertaken for cure. Carcinomas included grade 2 (G2) moderately differentiated (n=16) and grade 3 (G3) poorly differentiated (n=11) cases. Median age of patients at the diagnosis was 71 years (range 54–82). Staging of vulvar squamous cell carcinomas was performed according to the FIGO classification: 10 patients were stage 1A, six were stage 1B and 11 were stage IIIB. Normal vulvar tissues (n=12) were obtained from normal-appearing areas adjacent to non-neoplastic epithelial disorders. Only isolated non-neoplastic lesions, diagnosed in the absence of invasive squamous cell carcinomas, were included in the study. They included 10 cases of lichen sclerosus, 15 cases of squamous cell hyperplasia and 26 cases of VIN (19 VIN1 and seven VIN2). Two experienced pathologists (ET and GFZ) confirmed the histologic diagnosis of each vulvar lesion. None of the samples displayed morphological evidence of HPV infection.
Immunoperoxidase Detection of p27Kip1
All immunohistochemical analyses were performed on routinely processed, formalin-fixed, paraffin-embedded tissues employing an avidin–biotin complex immunoperoxidase technique, as previously described.15, 17. Briefly, successive 5 μm tissue sections were cut from blocks selected for the presence of representative lesions and mounted on charged and precleaned slides. For detection of p27Kip1 after incubation in an oven at 43°C for 12 h, sections were dewaxed and rehydrated. For antigen retrieval they were heated in a microwave oven for a total of 10 min at 750 W submerged in a citrate buffer (10 mmol/l, pH 6.0). Sections were then treated to block endogenous peroxidase and, after blocking with goat serum for 1 h at room temperature, the primary antibody was applied overnight at 4°C in a high-humidity chamber. After washing, immunostaining was performed using the Vectastain ABC and diaminobenzidine kits (Vector Laboratories, Burlingame, CA, USA), as described. Sections were then counterstained with 1% modified Harris hematoxylin, dehydrated and mounted.
A polyclonal anti-p27Kip1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a concentration of 1 μg/ml (in PBS with 10% goat serum) since this gave a good cellular staining with minimal background. As a negative control, a duplicate section of each tissue sample was immunostained in the absence of the primary antibody. A breast carcinoma with known positive immunostaining for p27Kip1 served as a positive control. A strong nuclear staining of lymphocytes provided a useful internal positive control for preservation of the p27Kip1 immunogenicity in most sections examined. The number of cells with positive reaction was calculated semiautomatically by means of a computer-assisted cellular image analyzer (Image Pro-Plus rel. 4.0, Media Cybernetic, Silver Spring, MA, USA), as previously reported.17 All scoring and interpretations of the results were made by two of the authors independently (AS and GFZ) without knowledge of other clinicopathological variables. The few cases with discrepant scoring were re-evaluated jointly on a second occasion, and agreement was reached. Positive and negative control slides were included within each batch of slides.
Immunoperoxidase Detection of 8-OHdG
Immunohistochemical detection of 8-OHdG was performed as previously described. Briefly, slides were washed twice with PBS, treated with RNase (100 μg/ml Sigma) 1 h at 37°C and washed again. They were subsequently treated with proteinase K (10 μg/ml, Sigma) at room temperature. In order to denature DNA, the samples were incubated with 4N HCl for 10 min and with 50mM TRIS Base for 5 min at room temperature, which is an essential step for adduct evaluation.18 After washing with PBS, the samples were incubated with 0.3% H2O2 in methyl alcohol at room temperature for 30 min to quench endogenous peroxidase activity. Nonspecific binding was blocked with 1.5% normal horse serum and incubation with the monoclonal antibody 1F712 diluted 1:50 in horse serum was performed overnight at +4°C. The slides were then incubated with a biotinylated horse anti-mouse secondary antiserum, and the reaction was visualized with the ABC complex followed by diaminobenzidine (Vector Laboratories, Burlingame, CA, USA). The samples were dehydrated in serial ethyl alcohol solutions and xylene and mounted. To minimize variations in the immunohistochemical assay, untreated control samples and 50 μg/ml aflatoxin B1-treated MCF-7 cells were stained together with each batch of samples, as previously reported.15 Negative staining was constantly obtained when slides were pretreated with DNase (Sigma) (100 μg/ml for 1 h at 37°C) before staining or were stained omitting primary antibody, or with the specific polyclonal antiserum preabsorbed with 8-OHdG (1 μg/μl for 20 min at room temperature) (data not shown).
Semiquantitative evaluation of the staining was performed as previously described measuring the relative intensity of nuclear staining in 100 randomly selected cells in the areas of interest, excluding stromal and inflammatory cells. The image was obtained in black and white and the average optical density was recorded. Results were expressed for each sample as the average of relative optical density multiplied by 1000. The mean value for negative samples was 74.8±28.6 (mean±s.d.) and positive samples always displayed a significantly higher staining (512±102). Since we observed a significant variability in the values obtained for both negative and positive samples amongst each batch, the results obtained were standardized to controls and stratified into three categories in term of staining intensity: weak, when staining was higher than negative control but <2 times it; moderate, when staining was higher than 2 × negative control but <50% of the value of the positive control; high, when staining was higher than 50% of the value of the positive control. Samples were also stratified in term of percentage of positive cells and a staining score was defined taking into account both intensity of staining and percentage of positive cells. To this aim, percentage of positive cells was classified as: 0=0%; 1=1–10%; 2=11–50% and 3=>50% positive cells. Similarly staining intensity was classified as: 1=weak; 2=moderate and 3=strong. Staining score was calculated as: % positive cells × staining intensity and was stratified as: negative=0; low=1; moderate=2–3 and strong≥3 (Table 2).
Statistical Analysis
The alternate Welch test was used to compare expression of the two variables in the different groups. The association between 8-OHdG and p27Kip1 staining and other variables were calculated using contingency table methods and tested for significance using the Fisher's exact χ2 test. All calculations were performed using the STATA 5.0 statistical software package (Stata Corporation, TX, USA) and the results were considered statistically significant if the P-value was <0.05.
Results
Progressive Reduction of p27Kip1 Expression in Vulvar Carcinogenesis
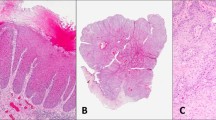
To investigate the significance of p27Kip1 in human vulvar carcinogenesis, the expression of this protein was evaluated by immunostaining in normal vulvar squamous epithelium, in non-neoplastic epithelial disorders of the vulva, such as lichen sclerosus and squamous cell hyperplasia, in VIN, and in invasive squamous cell carcinomas (Table 1 and Figure 1). In the absence of neoplasia, the normal vulvar epithelium displayed a clear staining for p27Kip1 in all the epithelial layers (Figure 1a).
Examples of p27Kip1 immunostaining in normal squamous epithelium (a), squamous cell hyperplasia (b), VIN (c), lichen sclerosus (d) and two cases (e and f) of invasive carcinomas of the vulva. (f) Shows a representative area of a vulvar squamous carcinoma with positive tumor cells. A progressive loss of staining is observed in the passage from normal to dysplastic to cancer lesions. When present, nuclear staining of lymphocytes (arrows) provided a useful internal positive control for preservation of the p27Kip1 immunogenicity. Original magnification, (a–e) × 200, (f) × 400.
In the presence of VIN, the percentage of reactive cells ranged from 0 to 100% with a median value of 100 (mean=77; s.d.=7). In some cases only cells of the intermediate and superficial layers stained positively (Figure 1c and data not shown). No significant differences were observed between VIN1 (range 60–100; mean=84; s.d.=10) and VIN2 (range 0–100; mean=75; s.d.=9) (data not shown).
p27Kip1 expression was further significantly decreased in invasive squamous cell carcinomas compared to VIN (P<0.0001). The percentage of reactive cells ranged from 0 to 100% with a median value of 75 (mean=64; s.d.=7) and staining was frequently heterogeneous both in terms of percent of positive cells and staining intensity (data not shown) (Table 1 and Figure 1e and f). Expression of p27Kip1 was observed in 22 out of the 27 (81%) primary vulvar carcinomas. When tumors were stratified according to tumor grade, three of 15 G2 (20%) and two of 11 G3 (18%) displayed no staining for p27Kip1 and no differences were observed in the percentage of positive cells between the two group of tumors (data not shown). One of the 16 (6%) stage 1 and three of the 11 (37%) stage III tumors displayed no staining for p27Kip1 and the mean percentage of positive cells was 74% (range 0–100) and 52% (range 0–100) in the stages I and III tumors, respectively, but these differences were not significant.
Interestingly, a different staining pattern for p27Kip1 was observed among the non-neoplastic epithelial disorders of the vulva included in this study (Figure 1b and d). Indeeed, squamous cell hyperplasia samples (n=15) displayed a strong positivity for p27Kip1 with the percentage of reactive cells ranging from 0 to 100% with a median value of 100 (mean=78; s.d.=11) which was significantly higher than squamous cell carcinomas (P=0.0002) but not VIN. On the other hand, lichen sclerosus lesions (n=10) displayed a reduced percentage of positive cells (range 0–100%) with a median value of 30 (mean=45; s.d.=15) which was significantly lower than squamous cell hyperplasia (P<0.0001), VIN (P<0.001) and cancer (P=0.004) (Table 1 and Figure 2f).
Examples of immunostaining for 8-OHdG in representative cases of normal squamous epithelium (a), squamous cell hyperplasia (b), VIN (c), lichen sclerosus (d) and invasive carcinoma (e) of the vulva. Most of cancer cells show a strong, positive staining compared with few cells stained positively in normal and non-neoplastic epithelial disorders. (f) Diagramatic representation of p27Kip1 and 8-OHdG expression levels in vulvar tumorigenesis. Shown are the mean±s.d. of the percentage of positive cells in squamous cell hyperplasia (SCH), lichen sclerosus (LS), VIN and squamous cell carcinoma (SCC) of the vulva. Original magnification, (a–d) × 200, (e) × 400.
Increased 8-OHdG Level in Vulvar Carcinogenesis
8-OHdG was evaluated by immunostaining in the same series of vulvar samples using the specific anti-8-OHdG monoclonal antibody 1F712, 15, 16 (Table 1 and Figure 2). The immunoperoxidase technique displayed no staining or only a weak sporadic staining for 8-OHdG adducts in the basal and parabasal layers of normal squamous epithelium of vulva (Figure 2a). The percentage of positive cells ranged from 0 to 30% with a median value of 10% (mean=25; s.d.=6) positive cells that was used as a cutoff to distinguish positive and negative samples. VIN were mostly negative with only 14 cases (54%) displaying a positive staining in at least 10% of cells. The percentage of positive cells was significantly lower in VIN1 (range 0–30; mean=8; s.d.=5; median=0) compared to VIN2 (range 0–10; mean=29; s.d.=8; median=15) lesions (P=0.003) (Figure 2c and data not shown).
Staining for 8-OHdG was further increased in invasive squamous cell carcinomas compared to VIN (Table 1 and Figure 2). The percentage of reactive cells ranged from 0 to 100% with a median value of 80 (mean=64; s.d.=37.3). The difference in the percentage of positive cells compared with VIN was highly significant (P≤0.0001) (Table 1). Staining in at least 10% of cells was observed in 21 out of the 27 (78%) primary vulvar carcinomas. The remaining six cases included four of 15 (27%) G2 and two of 11 (18%) G3 cancers. When tumors were stratified according to tumor grade, the mean percentage of positive cells was not significantly different between the two group of tumors (data not shown). No significant differences were also observed when tumors were stratified according to tumor stage (data not shown).
As for p27Kip1 a different staining pattern for 8OH-dG was observed among the non-neoplastic epithelial disorders of the vulva included in this study. In fact, an intermediate percentage of positive cells ranging from 0 to 75% with a median value of 10 (mean=28; s.d.=7) comparable to VIN2 but significantly higher than VIN1 (P<0.0001) and lower than squamous cell carcinomas (P<0.0001) was detected in squamous cell hyperplasia lesions (n=15) (Table 1 and Figure 2f). Lichen sclerosus lesions (n=10) displayed a higher percentage of positive cells ranging from 0 to 80% (mean=40; s.d.=10; median=50) which was significantly lower than squamous cell carcinomas (P=0.005) but higher than VIN (P=0.0007) and squamous cell hyperplasia (P=0.003) (Figure 2b–f and Table 1).
When results were stratified according to a staining score that takes into account both the percentage of positive cells and staining intensity, only seven (47%) of the squamous cell hyperplasia samples were scored as positive, with six (40%) of them displaying a strong staining (with positive cells ranging from 30 to 70%) and one (7%) showing a low-moderate staining (with up to 20% weakly positive cells). The percentage of positive cases increased progressively in VIN, lichen sclerosus and carcinoma samples. Fourteen out of 26 (54%) VIN and seven (70%) of lichen sclerosus were scored as positive. Finally, 22 out of 27 (81%) invasive carcinomas were scored as positive with 21 (77.8%) of them displaying a strong staining (Table 2).
Correlation between p27Kip1 and 8-OHdG Levels in Vulvar Lesions
The overall median value of positive cells in the vulvar lesions analyzed in this study was 90 and 50, respectively, for p27Kip1 and 8-OHdG. When these values were used as cutoff to distinguish between positive and negative cases, 34 (44%) cases were scored as positive for 8-OHdG expression and 44 (56%) as negative. A total of 17 cases positive for 8-OHdG (50%) were also positive for p27Kip1 expression and a similar number of cases (50%) were scored as negative for p27Kip1. In all, 27 cases negative for 8-OHdG (61%) were also negative for p27Kip1 expression and 17 (39%) were scored as positive for p27Kip1. Thus, low expression of p27Kip1 was more frequent in cases negative for 8-OHdG expression but the difference was not significant. Similar results were obtained when the same analysis was performed considering only the VIN and tumor samples together or separately (data not shown).
Discussion
Squamous cell carcinoma of the vulva is an important medical problem since it can affect urinary and sexual functions, threaten life and have profound psychological impacts on patients. Moreover, although it is traditionally seen in women in their 60s and 70s, incidence in much younger women is increasing and responsible factors are still unknown.1 Multiple sexual partners, a history of genital warts, pre-invasive cervical cancers, immunodeficiency and cigarette smoking have been reported as risk factors for vulvar tumorigenesis. Specific genital HPV, especially HPV16, have been also strongly implicated in the process and changes in the epidemiology of HPV infection in the past two decades might be responsible for the increasing incidence of invasive vulvar cancer in young women.1
However, limited research has been performed on the pathogenesis of vulvar carcinomas and cellular and molecular mechanisms responsible for the development and progression of neoplastic vulvar lesions remain unclear.
In this study, the expression of the CDK inhibitor p27Kip1 and the extent of oxidative DNA damage were analyzed in vulvar multistep carcinogenesis. Malignant cells are characterized by abnormal proliferation resulting from altered cell-cycle regulatory mechanisms and deregulation of cell-cycle machinery has been reported in gynecological malignancies, including vulvar cancer.19, 20 p27Kip1 is a negative regulator of cell-cycle progression acting as an inhibitor of cyclin/CDK complexes.17 It has been implicated in negatively regulating cell proliferation in response to extracellular signals and is induced upon serum deprivation. In normal epithelial cells increased expression of p27Kip1 mediates arrest of cells in the G1 phase of the cell cycle induced by different types of inhibitory signals.7 Although the p27Kip1 gene is only rarely mutated in cancer cells, reduced expression of the p27Kip1 protein has been observed in a variety of human malignancies and has been related to increased proteasome-dependent degradation of this protein.7, 21, 22 It is of interest that loss of p27Kip1 can be an early event in the multistep process of human tumorigenesis.7, 17 Progressive loss of p27Kip1 is commonly observed during progression from normal to benign and malignant tumors, and its decreased expression has been related with the acquisition of a metastatic potential and a poorer prognosis.7, 21, 22 Our results demonstrate that a decrease in p27Kip1 expression is associated with the development of vulvar cancer and may play an important role in the early stage of vulvar tumorigenesis. In fact, its expression was already reduced in pre-invasive neoplastic lesions (VIN) of the vulva and the decrease became progressively more evident in the passage from low VIN to carcinoma (Table 1 and Figure 1). In cancers, no differences were observed in the expression of p27Kip1 with advancing tumor grade and stage in our series of vulvar cancers thus further confirming the hypothesis that alterations in p27Kip1 expression likely play a role in the early phases of vulvar tumorigenesis.
The decrease in p27Kip1 expression in vulvar carcinomas is in agreement with similar findings in several other types of human malignancies7 and with previous reports regarding vulvar cancers.6, 8 Indeed, p27Kip1 expression was already shown to be detectable only in a small percentage of vulvar cancers6, 8 but limited data are available on preneoplastic vulvar lesions.6 In this study, we evaluated the expression of p27Kip1 on lesions representing various phases of the multistep human tumorigenesis and demonstrated that reduction/loss of p27Kip1 expression is an early event in the process being already evident in non-neoplastic epithelial disorders and VIN and increasing progressively up to invasive cancer (Figures 1 and 2f). A reduced expression of p27Kip1 might simply reflect an increased proliferation rate of tumor cells since p27Kip1 is usually expressed at higher level in resting cells. However, no relationship was observed between expression of p27Kip1 and proliferative activity in different types of cancers suggesting that deregulated expression of p27Kip1 might contribute to tumor formation through mechanisms other than increased cell proliferation.7, 23 Taken together with the results of previous studies, our data confirm the potential important role of loss of p27Kip1 expression in the development and progression of vulvar lesions and suggest that it likely represents a primary event in human vulvar carcinogenesis. It is noteworthy that in a previous study in which we analyzed the same molecular markers in cervical tumorigenesis, we observed a more dramatic reduction of p27Kip1 expression in cervical diseases than the one observed in vulvar lesions.17 These findings support the hypothesis of a different molecular pathway for vulvar compared to cervical tumorigenesis and HPV might play a role in this phenomenon. In fact, HPV infection is present in the majority of cervical lesions24 while it was never observed in our series of old patients with vulvar lesions. It is noteworthy that HPV infection has been reported to be responsible of the increased incidence of vulvar cancers in the last decades, especially in young women.1 Thus, it is possible that different results might be obtained by analyzing HPV-related vulvar lesions in young women. Studies are ongoing to verify this hypothesis.
DNA base modification is one of the major effects of free radical attack upon cellular DNA and oxidation of the C8 of guanine, with formation of 8-OHdG, is one of the most abundant types of oxidative DNA damage. The presence of 8-OHdG residues in DNA leads to GC → TA transversion unless repaired prior to DNA replication.11 Although numerous pieces of evidence suggest a direct relation between 8-OHdG formation and carcinogenesis in vivo,25 it is still debated whether elevated levels of 8-OHdG play a causative role in carcinogenesis or are merely the result of the disease. However, the observation that several carcinogens cause oxidative DNA damage, with increased 8-OHdG levels, in laboratory animals before tumor formation occurs and that tumor progression is linked to this type of damage strongly support the hypothesis that oxidative DNA base modifications play an important role in carcinogenesis.26
To our knowledge, this is the first study investigating the expression level of 8-OHdG in multistep vulvar carcinogenesis. We observed a progressive increase in the levels of 8-OHdG from normal to VIN to invasive carcinomas (Figure 2 and Tables 1 and 2). We previously obtained similar results in cervical tumorigenesis demonstrating a significant association between increase in the level of oxidative DNA damage and progression of preinvasive cervical lesions.17 The significance of this finding is unknown. It could simply reflect an alteration in the regulation of redox balance in precancerous cells compared with normal cells and this alteration might become progressively more important during the process of tumor development (ie in the passage from VIN to cancer). However, it cannot be excluded a priori that oxidative DNA damage, and oxidative stress in general, play an important role in vulvar tumorigenesis and might itself promote vulvar cancer development. Further studies will be needed to confirm this hypothesis and to identify the major causes of oxidative stress in vulvar cells. We previously hypothesized that HPV viral infection might be an important cause of oxidative stress in cervical lesions.17 This is probably not the case in our series of vulvar cancer since the age of patients and the absence of morphological signs of HPV infection suggests that HPV infection is unlikely to be involved in the development of these lesions. Thus, other factors are probably involved and cigarette smoke and diet might well play a role. In fact, smoke constituents have been found in cervical mucus and it has been hypothesized that chemicals present in cigarette smoke may modify important cellular genes and thus provide an additional step in tumor progression although the underlying biological mechanisms have not been identified.27 Several constituents of cigarette smoke have a pro-oxidative activity and we believe that smoking might promote cervical as well as vulvar carcinogenesis by inducing oxidative DNA damage.
However, other still unknown factors, including aging, might contribute to oxidative DNA damage in vulvar cells. Moreover, it is noteworthy that susceptibility to the DNA-damaging activity of pro-oxidant agents, including carcinogens, is strictly dependent on individual genetic polymorphisms in several metabolic enzymes.28 Thus, evaluation of 8-OHdG levels is a valuable marker of a high-risk condition since it takes into account both genetic variability and enviromental factors (such as smoking, virus infections and sexual habits) which contribute to determining the level of oxidative damage in vulvar cells. Further studies will be needed to identify all the factors and molecular mechanisms involved.
We did not find any significant association between expression of p27Kip1 and the levels of 8-OHdG in vulvar lesions (Figure 2f). This finding might simply further confirm the importance of both events in vulvar tumorigenesis. However, further studies will be needed to definitively assess whether DNA-oxidative damage plays a causal role in vulvar carcinogenesis or is merely an epi-phenomenon of cell transformation.
An interesting result of this study is that a reduced expression of p27Kip1 and an increase in DNA-oxidative damage were already observed in non-neoplastic epithelial disorders at an intermediate level compared to what observed in neoplastic lesions (Table 1 and Figure 2f). A surprising finding was that the occurrence of these alterations in lichen sclerosus lesions was more similar to what observed in carcinoma samples than in other non-neoplastic epithelial lesions. This result is in agreement with previous data on the occurrence of molecular alterations in squamous cell carcinomas and other preneoplastic vulvar lesions. Thus, expression of cyclin D1 was shown to be overexpressed in 51% of squamous cell carcinomas and in 50% of lichen sclerosus but only in 31% of SCH.3 Similarly, loss of pRb expression was shown to occur in lichen sclerosus at a percentage (40%) more similar to the one observed in squamous cell carcinomas (37%) than in SCH (62%).3 Similar results have been also reported for p53 expression and mutations29, 30 and a similar trend was previously observed for COX-2 expression.31 The significance of this finding is unclear. It might suggest that lesions with histological appearance of lichen sclerosus have to be considered more advanced and at higher risk of progression than other non-neoplastic epithelial disorders. On the other hand, it might also suggest that lichen sclerosus represents a more ‘active’ lesion, with more evident signs of alterations, than squamous cell hyperplasia (also referred to as ‘chronicus lichen simplex’).32, 33
In conclusion, we believe that the results of the present study suggest that both loss of p27Kip1 expression and DNA-oxidative damage are separately involved in the development and progression of vulvar diseases and changes in the expression of both parameters occur at an early stage of malignant transformation. Further studies on the expression of these two markers in a larger series of vulvar lesions, including VIN3 lesions, are warranted to evaluate whether they might serve as markers of risk progression of preneoplastic vulvar lesions and to better understand their significance in terms of vulvar cancer development and of their association with the biological and clinical behavior of these cancers.
References
Joura E . Epidemiology, diagnosis and treatment of vulvar intraepithelial neoplasia. Curr Opin Obstet Gynecol 2002;14:39–43.
Wilkinson E, Xie D-L . Benign disease of vulva. In: Kurman RJ (ed.). Blaustein's Pathology of the Female Genital Tract, 5th edn. Springer Verlag: New York, NY, 2002.
Rolfe K, Eva L, MacLean A, et al. Cell cycle proteins as molecular markers of malignant change in vulvar lichen sclerosus. Int J Gynecol Cancer 2001;11:113–118.
Chan May K, Cheung T, Chung T, et al. Expression of p16INK4 and retinoblastoma protein Rb in vulvar lesions of Chinese women. Gynecol Oncol 1998;68:156–161.
Lerma E, Matias-Guiu X, Lee J, et al. Squamous cell carcinoma of the vulva: study of ploidy, HPV, p53, and pRB. Int J Gynecol Pathol 1999;18:191–197.
Zamparelli A, Masciullo V, Bovicelli A, et al. Expression of cell-cycle-associated proteins pRB2/p130 and p27kip1 in vulvar squamous cell carcinomas. Hum Pathol 2001;32:4–9.
Sgambato A, Cittadini A, Faraglia B, et al. Multiple functions of p27Kip1 and its alterations in tumor cells: a review. J Cell Physiol 2000;183:18–27.
Knopp S, Bjørge T, Nesland J, et al. p16INK4a and p21Waf1/Cip1 expression correlates with clinical outcome in vulvar carcinomas. Gynecol Oncol 2004;95:37–45.
Olinski R, Gackowski D, Foksinski M, et al. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med 2002;33:192–200.
Dizdaroglu M . Oxidative damage to DNA in mammalian chromatin. Mutat Res 1992;275:331–342.
Grollman AP, Moriya M . Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet 1993;9:246–249.
Yin B, Whyatt RM, Perera FP, et al. Determination of 8-hydroxy-deoxyguanosine by immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic Biol Med 1995;18:1023–1032.
Yarborouh A, Zhang Y-J, Hsu T-M, et al. Immunoperoxidase detection of 8-hydroxydeoxyguanosine in aflatoxin B1 treated rat liver and human oral mucosa cells. Cancer Res 1996;56:683–688.
Santella RM . Immunological methods for detection of carcinogen-DNA damage in humans. Cancer Epidemiol Biomarkers Prev 1997;216:166–171.
Romano G, Sgambato A, Mancini R, et al. 8-hydroxy-2-deoxyguanosine in cervical cells: correlation with grade of dysplasia and human papillomavirus infection. Carcinogenesis 2000;21:1143–1147.
Romano G, Sgambato A, Flamini G, et al. Evaluation of 8-hydroxydeoxyguanosine in human oral cells: the importance of tobacco smoke and urban enviroment. Anticancer Res 2000;20:3804–3806.
Sgambato A, Zannoni G, Faraglia B, et al. Decreased expressions of the CDK inhibitor p27 kip 1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol Oncol 2004;92:776–783.
Al-Atrash J, Zhang YJ, Lin D, et al. Quantitative immunohistochemical analysis of 4-ABP-DNA adducts in cultured cells and mice: comparison with gas chromatography/mass spectroscopy analysis. Chem Res Toxicol 1995;87:747–752.
Milde-Langosch K, Riethdorf S . Role of cell-cycle regulatory proteins in gynecological cancer. J Cell Physiol 2003;196:224–244.
Sanseverino F, Torricelli M, Petraglia F, et al. Role of the retinoblastoma family in gynecological cancer. Cancer Biol Ther 2003;2:636–641.
Lee MH, Yang HY . Negative regulators of cyclin-dependent kinases and their roles in cancers. Cell Mol Life Sci 2001;58:1907–1922.
Nevins JR . The Rb/E2F pathway and cancer. Hum Mol Genet 2001;10:699–703.
Sgambato A, Migaldi M, Faraglia B, et al. Cyclin D1 expression in papillary superficial bladder cancer: its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer 2002;97:671–678.
Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244–265.
Feig DI, Reid TM, Loeb LA . Reactive oxygen species in tumorigenesis. Cancer Res 1994;54 (Suppl):1890s–1894s.
Malins DC, Polissar NL, Gunselman SJ . Progression of human breast cancers to the metastatic state is linked to hydroxyl radical-induced DNA damage. Proc Natl Acad Sci USA 1996;93:2557–2563.
Hellberg D, Nilsson S, Haley N, et al. Smoking and cervical intraepithelial neoplasia: nicotine and cotinine in serum and cervical mucus in smokers and nonsmokers. Am J Obstet Gynecol 1988;158:910–913.
Clapper ML . Genetic polymorphism and cancer risk. Curr Oncol Rep 2000;2:251–256.
Carlson J, Amin S, Malfetano J, et al. Concordant p53 and mdm-2 protein expression in vulvar squamous cell carcinoma and adjacent lichen sclerosus. Appl Immunohistochem Mol Morphol 2001;9:150–163.
Rolfe K, MacLean A, Crow J, et al. p53 mutations in vulval lichen sclerosus adjacent to squamous cell carcinoma of the vulva. Br J Cancer 2003;89:2249–2253.
Ferrandina G, Ranelletti F, Salutari V, et al. Expression of cyclooxygenase-2 (COX-2) in non-neoplastic and neoplastic vulvar epithelial lesions. Gynecol Oncol 2004;92:537–544.
Kiryu H, Ackerman A . A critique of current classifications of vulvar diseases. Am J Dermatopathol 1990;12:377–392.
Scurry J . Does lichen sclerosus play a central role in the pathogenesis of human papillomavirus negative vulvar squamous cell carcinoma? The itch-scratch-lichen sclerosus hypothesis. Int J Gynecol Cancer 1999;9:89–97.
Acknowledgements
We are grateful to Prof Regina Santella (Mailman School of Public Health, Columbia University, New York, USA) for providing the 1F7 antibody and for helpful advices. This work was supported in part by grants from the Università Cattolica (to AC, AS and GFZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zannoni, G., Faraglia, B., Tarquini, E. et al. Expression of the CDK inhibitor p27kip1 and oxidative DNA damage in non-neoplastic and neoplastic vulvar epithelial lesions. Mod Pathol 19, 504–513 (2006). https://doi.org/10.1038/modpathol.3800532
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800532
Keywords
This article is cited by
-
Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients
Journal of Experimental & Clinical Cancer Research (2012)