Abstract
We identified 14 B-cell neoplasms with concurrent t(14;18) and chromosome 8q24 or c-MYC translocations shown by conventional cytogenetics or fluorescence in situ hybridization analysis. All cases assessed by conventional cytogenetics had a complex karyotype. There were 10 men and four women, with a median age of 55 years (range, 29–72). None of these patients had a history of follicular lymphoma. The biopsy specimens were obtained from bone marrow, lymph node, and extranodal sites. Morphologically, nine neoplasms had features of Burkitt or atypical Burkitt lymphoma/leukemia and three were diffuse large B-cell lymphoma with high-grade cytologic features. The remaining two cases were plasmablastic myeloma and low-grade B-cell lymphoma, respectively. All cases expressed BCL-2. The proliferation index assessed by using Ki-67 (MIB1) was 5% in the low-grade B-cell lymphoma, 80% in the plasmablastic myeloma, 90–95% in three cases of diffuse large B-cell lymphoma, and ranged from 90 to >99% in most Burkitt and atypical Burkitt neoplasms. The patient with low-grade B-cell lymphoma was treated with rituximab. All other patients received intensive combination chemotherapy. Two of these patients underwent bone marrow transplantation, and one patient received radiation therapy in addition to transplantation. The median follow-up period was 9 months (range, 3–81). In all, 10 patients died with a median survival of 9 months (range, 3–81). We conclude that most B-cell lymphomas with concurrent t(14;18) and 8q24/c-MYC translocations fall within the morphologic spectrum of diffuse large B-cell and Burkitt lymphoma. These neoplasms are high-grade and are associated with a poor prognosis. However, this combination of molecular abnormalities can also rarely occur in other neoplasms, such as the cases of low-grade B-cell lymphoma and plasmablastic myeloma in this study.
Similar content being viewed by others
Main
The t(14;18)(q32;q21) is typically found in lymphoid neoplasms of follicle center cell lineage. Approximately 80–90% of follicular lymphomas and 20–30% of diffuse large B-cell lymphomas carry this translocation.1, 2 As a result of the t(14;18), the BCL2 gene at 18q21 is juxtaposed with the immunoglobulin heavy chain (IGH) gene on the derivative chromosome 14, resulting in overexpression of BCL-2, an antiapoptotic molecule.
The c-MYC gene at 8q24 is involved in three translocations, most commonly t(8;14)(q24;q32), and less often t(2;8)(p12;q24) and t(8;22)(q24;q11).1, 2, 3 Via these translocations, the c-MYC gene is juxtaposed with the IGH gene on the derivative chromosome 14, or the immunoglobulin light chain genes are juxtaposed with c-MYC on the derivative chromosome 8. This results in overexpression of c-MYC, driving cell proliferation and expression of other genes involved in cell growth.4 C-MYC translocations are characteristic of Burkitt lymphoma, with the t(8;14) occurring in 80–90% of cases.3, 4 However, c-MYC translocations also have been reported, although rarely, in cases of transformed follicular lymphoma, blastoid variant of mantle cell lymphoma, chronic lymphocytic leukemia in prolymphocytoid transformation, and de novo prolymphocytic leukemia.5, 6, 7, 8, 9
In this report, we discuss 14 lymphoid neoplasms in which both the t(14;18) and chromosome 8q24 or c-MYC translocations were detected. None of these patients had a history of follicular lymphoma. We reviewed the histologic findings, assessed the proliferation index and the status of BCL-2 expression by immunohistochemical analysis in each tumor, and correlated the findings with clinical outcome.
Materials and methods
Case Selection
The files of the Cytogenetics Laboratory of the Department of Hematopathology at The University of Texas MD Anderson Cancer Center were searched for cases that had concurrent t(14;18)(q32;q21) and 8q24 translocations as detected by conventional cytogenetic analysis and/or t(14;18) and c-MYC rearrangement detected by fluorescence in situ hybridization (FISH) analysis. The search was limited to cases accessioned during July 1998–May 2005. Clinical information was obtained by review of corresponding medical records.
Morphologic Analysis
Hematoxylin–eosin-stained histologic sections of bone marrow aspirate clot and core biopsy, lymph node biopsy, and extranodal biopsy specimens were reviewed. Peripheral blood and bone marrow aspirate smears stained with Wright–Giemsa also were reviewed. Complete blood cell and differential counts were performed on peripheral blood smears of all patients.
Flow Cytometry Immunophenotyping
Bone marrow aspirates, peripheral blood or a cell suspension of tissue biopsy specimens were assessed by using standard 3- or 4-color flow cytometry immunophenotypic analysis and either a FACScan or FACSCalibur cytometer (Becton-Dickinson Biosciences, San Jose, CA, USA) as described previously.8 Incubation of cells with monoclonal antibodies at 4°C was followed by red blood cell lysis with phosphate-buffered saline solution. Cells were then resuspended and fixed with 1% formaldehyde. Lymphocytes were gated for analysis using CD45 expression and side scatter. The CD45 antibody used for gating was conjugated to peridin chlorophyll alpha protein. Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated antibodies were used as negative controls, and cursors were set to include >95% of events as negative. For the purpose of this study, expression of an antigen by 20% or more of cells was defined arbitrarily as being positive. The panel of antibodies included CD3, CD4, CD5, CD7, CD10, CD13, CD19, CD20, CD23, CD33, CD34, CD38 and CD138, terminal deoxynucleotidyl transferase (TdT), and immunoglobulin κ and λ light chains. In the case of low-grade B-cell lymphoma, the panel included CD11c, CD79b, CD23, and FMC7. All antibodies were obtained from Becton-Dickinson Biosciences, except for TdT (Supertech, Bethesda, MD, USA).
Immunohistochemistry
Immunohistochemical studies were performed on formalin-fixed, paraffin-embedded tissue sections of bone marrow aspirate clot and core biopsy, lymph node biopsy, or extranodal biopsy specimens. After deparaffinization and dehydration of the sections in graded alcohols and xylene, endogenous peroxidase was blocked with hydrogen peroxide. Heat-induced epitope retrieval was performed using citrate buffer, pH 6.0. The monoclonal antibodies used were specific for CD5 (1:20), CD10 (1:70), CD20 (1:40), BCL-2 (1:200), Ki-67 (MIB-1, 1:100), and myeloperoxidase (1:1000) (DAKO, Carpinteria, CA, USA); and cyclin D1 (1:50, LabVision, Fremont, CA, USA), as previously described.9 In the case of low-grade B-cell lymphoma, we used Ki-67 and CD20 double staining to assess the proliferation rate of the neoplastic cells.
Conventional Cytogenetic Studies
Conventional G-band karyotype analysis was performed on 10 cases of bone marrow aspirate material, one case of small intestinal tissue and one case of testicular tissue, using methods previously described.10 The karyotypes were reported according to the 1995 International System for Human Cytogenetic Nomenclature.11
Fluorescence In Situ Hybridization
FISH analysis was performed in 12 cases using an LSI MYC dual-color breakapart rearrangement probe, a mixture of two probes that hybridize to opposite sides of the region located 3′ of MYC (spectrum orange on the centromeric side and spectrum green on the telomeric side of the MYC gene breakpoint) (Vysis, Downers Grove, IL, USA). An LSI IGH/BCL-2 dual-color, dual-fusion translocation probe, which detects juxtaposition of the IGH locus with BCL2 gene sequences (LSI IGH probe labeled with spectrum green and LSI BCL-2 probe labeled with spectrum orange) (Vysis), was used in a subset of cases. The FISH assay was performed by using either a freshly dropped slide from a harvested bone marrow aspirate specimen or a G-banded slide for metaphase mapping according to the manufacturer's instructions. Hybridization signals were analyzed by using a fluorescent microscope (Carl Zeiss, Thornwood, NY, USA) with appropriate filters. The images were captured using the Cyto Vision imaging system (Applied Imaging, Santa Clara, CA, USA). The assay was considered positive for c-MYC rearrangement if the signals were one orange, one green, and one yellow (fusion), and positive for t(14;18) if the signals were one orange, one green, and two yellow (fusion).
In situ hybridization analysis for EBV encoded RNA was performed on formalin-fixed paraffin-embedded tissue sections of the plasmablastic neoplasm using a peptide nuclear acid in situ hybridization kit (Dako) according to the manufacturer's instructions with the appropriate positive and negative controls.
Results
Clinical Findings
The clinical data for the 14 patients are summarized in Table 1. There were 10 men and four women, with a median age of 55 years (range, 29–72). None of the patients had peripheral lymphadenopathy or hepatosplenomegaly. Nine patients (cases 1–2, and 7–13) presented with a mass or masses involving extramedullary sites. Seven patients (cases 3–6, 8, 11, and 14) presented with an elevated leukocyte count with circulating abnormal lymphoid cells. Two of these patients (cases 8 and 11) also had extramedullary tumors. Neoplastic cells were detected in the cerebrospinal fluid in five patients, three patients (cases 3, 6 and 7) at the time of initial diagnosis and two patients (cases 8 and 9) during the course of disease. The patient with myeloma presented with bone pain and radiologic examination revealed lytic lesions of the skull, pelvis, and femurs. In the patient with low-grade B-cell lymphoma (case 14), staging by computerized axial tomography (CAT) scan revealed mild retroperitoneal and mesenteric lymphadenopathy of up to 1 cm, and no evidence of lymphadenopathy involving the mediastinal, hilar or axillary regions. The retroperitoneal and mesenteric lymph nodes were not biopsied.
All patients (except for case 14) had an elevated serum lactate dehydrogenase level ranging from 667 to over 42 000 IU/l, with a median of 1600 IU/l (normal range: 313–618 IU/l). In the seven patients with circulating lymphoma cells found in peripheral blood, the leukocyte count ranged from 11.4 to 137.9 × 109/l (median: 17.5 × 109/l) (normal range: 4–11 × 109/l), and the percentage of the abnormal lymphoid cells ranged from 10 to 79% (median: 44%).
All patients, except for case 14, received intensive combination chemotherapy following admission, and two patients underwent bone marrow transplantation (cases 10 and 13). Case 10 was also given radiation therapy. Case 14 received rituximab alone for low-grade B-cell lymphoma. Six patients received chemotherapy prior to their admission to our hospital. In all, 10 patients died, with a median survival of 9 months (range, 3–81). Four patients (cases 1, 5, 12 and 14) are alive, 7, 11, 5, 7 months after initial diagnosis, respectively, at the time of writing. The patient with low-grade B-cell lymphoma achieved remission with normalization of the peripheral blood leukocyte count and no evidence of monoclonal B cells as assessed by flow cytometry. This patient is currently off therapy and is being observed.
Morphologic Findings
Bone marrow specimens were evaluated in all 14 patients and nodal or extranodal biopsy specimens were available in nine patients. Nine cases had morphologic features most consistent with Burkitt or atypical Burkitt lymphoma. Three cases were diffuse large B-cell lymphoma with high-grade cytologic features. There was also one case of plasmablastic myeloma and one case of low-grade B-cell lymphoma.
The bone marrow core biopsy specimens in 11 cases revealed involvement by lymphoma/leukemia. The cellularity ranged from 40 to 90%, with a focal or diffuse interstitial infiltrate of abnormal lymphoid cells.
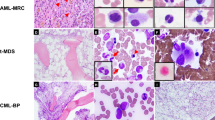
In the cases with features of Burkitt (n=4) or atypical Burkitt lymphoma/leukemia (n=5), the bone marrow aspirate smears showed intermediate-sized lymphoid cells with basophilic cytoplasm, round to slightly oval nuclei (Burkitt lymphoma) or slightly irregular nuclei (atypical Burkitt) with variably clumped chromatin, and single to multiple prominent nucleoli. Cytoplasmic vacuoles were present. The neoplastic cells ranged from 49 to 96% (Figure 1).
Biopsy specimens were obtained from lymph nodes (n=4; pelvic, mesenteric, and retroperitoneal) or extranodal sites (n=13; small intestine, ileocecal valve, colon, omentum, prostate, breast, testes, chest wall, and lip). Histologic sections of the nodal and extranodal biopsy specimens in cases of Burkitt or atypical Burkitt lymphoma/leukemia showed a diffuse infiltrate of intermediate-sized, small noncleaved lymphoid cells with basophilic cytoplasm, irregular nuclei, and prominent nucleoli. Mitotic figures and apoptotic bodies were increased. A prominent starry-sky pattern with scattered tingible body macrophages was apparent (Figure 2). Cases of diffuse large B-cell lymphoma showed larger, vesicular and more irregular nuclei and larger nucleoli (Figure 3).
In the case of plasmablastic myeloma, the bone marrow aspirate smears showed medium-sized to large plasma cells with immature chromatin and scant amount of basophilic cytoplasm. The neoplastic cells had prominent nucleoli in the bone marrow biopsy specimen (Figure 4a–c).
(a–c) (Case 13): (a) Plasmablastic myeloma in bone marrow core biopsy shows sheets of large atypical cells with prominent nucleoli (hematoxylin and eosin, × 500). (b) Immature plasma cells have blastoid chromatin and scant amount of cytoplasm (Wright–Giemsa, × 1000). (c) High Ki-67 labeling of the tumor cells ( × 500).
In the case of low-grade B-cell lymphoma, the bone marrow core biopsy specimen demonstrated an interstitial infiltrate of small lymphocytes, representing approximately 50% of the cellularity (Figure 5a). The peripheral blood and bone marrow aspirate smears showed small to medium-sized lymphocytes with moderate cytoplasm, round nuclei with condensed chromatin and small but visible nucleoli (Figure 5b).
(a, b) (Case 14): (a) Low-grade B-cell lymphoma/leukemia involving bone marrow (hematoxylin and eosin, × 500). The neoplastic cells infiltrate in an interstitial pattern. (b) Peripheral blood smear shows that the neoplastic cells are of small size with visible to inconspicuous nucleoli, and scant amount of cytoplasm (Wright–Giemsa, × 1000).
Immunophenotypic Findings
Flow cytometric immunophenotypic analysis was performed in 13 cases and all neoplasms were of B-cell lineage. All 11 cases of high-grade B-cell lymphoma were positive for CD10, CD19, and CD20, and negative for myeloid and T-cell markers. Eight of the neoplasms were positive for monotypic surface immunoglobulin light chain (κ 5 and λ 3). In the other three cases there was no apparent surface light chain expression or restriction and the neoplastic cells were also negative for CD34 and TdT. The case of plasmablastic myeloma was positive for CD38, CD138, CD33, and cytoplasmic monotypic lambda light chain, and negative for CD13, CD20, CD34, CD56, CD117, and TdT. Flow cytometry immunophenotyping performed on the peripheral blood of the case of low-grade B-cell lymphoma was positive for CD10, CD19, CD20, CD22, CD23 (partial), CD43, CD79b, FMC7 (partial), surface IgM/D, and κ light chain, and was negative for CD5 and CD11c.
Immunohistochemical studies were performed on bone marrow core biopsy specimens and nodal or extranodal biopsy specimens, including case 12 that was only assessed by immunohistochemistry. The neoplastic cells in all cases of B-cell lymphoma were positive for CD20, CD10, and BCL-2, and were negative for CD5 and myeloperoxidase. The case of plasmablastic myeloma was positive for CD138, cyclin D1, and BCL-2. The proliferation index (Ki-67 labeling) was approximately 80% in the case of plasmablastic myeloma, 5% in the case of low grade B-cell lymphoma, and ranged from 90 to 95% in three cases of diffuse large B-cell lymphoma. In cases with morphologic features of Burkitt or atypical Burkitt lymphoma/leukemia, the proliferation rate was approximately 70% in one case, 80% in a second case, and ranged from 90 to >99% in the other neoplasms (Figure 6a and b). Further review of the two cases with <90% Ki-67 labeling found that tumors showed necrosis and markedly increased apoptotic bodies, and thus Ki-67 may underrepresent the proliferation rate in these cases.
Cytogenetic Findings
Conventional cytogenetic analysis was performed in 12 cases, with two cases of atypical Burkitt lymphoma not assessed (cases 11 and 12). All cases had a complex karyotype with multiple abnormalities (Table 2) (Figure 7a). Each case carried the t(14;18)(q32;q24). In addition, five cases showed t(8;14)(q24;q32), six cases showed t(8;22)(q24;q11), and one case showed t(2;8)(p11;q24). The plasmablastic myeloma and low-grade B-cell lymphoma carried the t(8;14)(q24;q32).
Fluorescence In Situ Hybridization
FISH analysis was performed in 12 cases using bone marrow aspirate smears (cases 6–11, 13), peripheral blood smears (cases 4 and 14), intestinal biopsy specimens (cases 1 and 12), and a lung biopsy specimen (case 2). Cases 3 and 5 were not assessed. In all seven cases assessed for both c-MYC rearrangement and IGH/BCL2, both abnormalities were identified. Three cases were assessed only for c-MYC rearrangement; all were positive. Two other cases were assessed only for IGH/BCL2 gene rearrangement and both were positive (Table 2).
In the case of low-grade B-cell lymphoma, a total of 200 interphases were analyzed for IgH/BCL2 fusion and a positive signal was found in 77.5% of the interphases. In a separate FISH analysis of 200 interphases for c-MYC rearrangement, a split signal was found in 50 interphases, demonstrating that 25% of analyzed cells harbored c-MYC rearrangement. An additional 31% of the cells had trisomy 8.
We performed in situ hybridization for Epstein–Barr virus-encoded RNA (EBER) on the biopsy sections of the plasmablastic myeloma and showed that the neoplastic cells were negative for EBER.
Discussion
In this report, we describe 14 lymphoid neoplasms in which conventional cytogenetics and/or FISH analysis revealed concurrent t(14;18) and either chromosome 8q24 or c-MYC rearrangement. In the 12 cases studied by conventional cytogenetics, these translocations were present as part of a complex karyotype. All cases presented de novo without a history of follicular lymphoma. Nine cases were high-grade neoplasms with morphologic features of Burkitt or atypical Burkitt lymphoma/leukemia, and three were diffuse large B-cell lymphoma. One case was plasmablastic myeloma, and other case was low-grade B-cell lymphoma.
Few studies of lymphoid neoplasms carrying both the t(14;18) and c-MYC translocations have been reported in the literature. The largest study published to date included 12 cases, six cases of t(14;18) with t(8;14), and six cases of t(14;18) with t(8;22).12 These patients presented with advanced disease and had a poor prognosis, and six cases had a history of follicular lymphoma. Thangavelu et al13 reported six cases with t(14;18) and 8q24 translocation: three that had t(8;14), and three that had t(8;22). Two of the six patients had a history of follicular lymphoma. There have been 11 case reports of Burkitt-type leukemia (ALL-L3) in the literature with both the t(14;18) and variants of c-MYC (t(8;14) or t(8;22)).13, 14, 15, 16, 17, 18 By contrast, concurrent t(2;8) and t(14;18) is very rare and has been reported only once in a cell line created from a patient with chronic lymphocytic leukemia.19 In this case, an immortalized cell line initially carried t(14;18) without t(2;8). The emergence of t(2;8) was presumed to be a secondary cytogenetic event that occurred in culture.
A novel finding in this study is the detection of the t(14;18) with 8q24/c-MYC rearrangement in cases of plasmablastic myeloma and low-grade B-cell lymphoma. This combination of translocations has not been reported in these neoplasms. The presence of c-MYC rearrangement has been reported in 15% of cases of multiple myeloma or primary plasma cell leukemia in a study by Avet-Loiseau et al.20 Although plasmablastic myeloma and plasmablastic lymphoma can be indistinguishable by immunophenotype and morphology, the strong cyclin D1 positivity and absence of Epstein–Barr virus support the diagnosis of plasmablastic myeloma over plasmablastic lymphoma in this case.21 In the low-grade B-cell lymphoma, we demonstrated a higher percentage of cells showing IGH/BCL2 fusion signals than c-MYC rearrangement, raising the possibility of c-MYC rearrangement as a secondary event. However, as the FISH analyses were performed on two separate peripheral blood smears, and the conventional cytogenetics showed all of the evaluated metaphases contained either both abnormalities, or neither abnormality, we were unable to confirm this possibility.
The t(14;18)(q32;21) is known to result in overexpression of BCL-2. BCL-2 overexpression is known to increase cell survival by preventing apoptosis. The t(14;18), by prolonging cell survival, predisposes the cell to the acquisition of secondary genetic aberrations. Overexpression of c-MYC increases apoptosis, promotes sustained cell proliferation and retards differentiation. Cooperation of BCL-2 and c-MYC thereby contribute to multistep lymphomagenesis, as has been demonstrated in vivo in transgenic mouse models.22 It has been shown that transgenic mice that overexpress BCL-2, after a latent period of 15 months, can then acquire c-MYC rearrangement at which time aggressive lymphomas develop.23
Although the histologic features of nine cases in this study were those of Burkitt or atypical Burkitt lymphoma/leukemia, with a starry-sky pattern, intermediate-sized cells, and a high proliferation rate, these neoplasms had unusual features. Each neoplasm was positive for BCL-2. As stated in the World Health Organization (WHO) classification,1 Burkitt lymphoma is usually BCL-2 negative. The Ki-67 results were also unusual in that some cases had a proliferation index less than the >99% described in the WHO classification. As these tumors were actively undergoing apoptosis/necrosis, the lower Ki-67 labeling index may be attributed to poor cell viability. Nonetheless, the BCL-2 expression and Ki-67 results may lead one to conclude that these neoplasms should not be classified as Burkitt or atypical Burkitt lymphoma/leukemia. For this study, we classified these neoplasms on the basis of their morphologic findings. As all nine patients had a poor outcome, despite aggressive therapy, this may be a semantic argument. Perhaps a practical approach is to designate these neoplasms as high-grade B-cell lymphoma not otherwise specified and perform cytogenetic and molecular studies to confirm the presence of c-MYC rearrangement.
As reflected in the literature, when a variant c-MYC translocation is present, t(8;22) is clearly more common than t(2;8). The reason for this association is unknown, and the one case of t(2;8) with t(14;18) is, to our knowledge, the first neoplasm described in the literature. It has been hypothesized that t(14;18) occurs as the primary event, followed by acquisition of t(8;14) or t(8;22) as the neoplasm progresses to blastic transformation.24 Martin-Subero et al25 have shown in two patients with t(14;18)-positive germinal center derived B-cell lymphomas in which amplification of c-MYC occurred with disease progression and correlated with poor clinical outcome and therapy resistance. In this study, it was not possible to discern whether these dual translocations developed sequentially or simultaneously. The presence of BCL-2 expression in all cases, however, suggests that t(14;18) preceded c-MYC rearrangement.
In conclusion, the clinical, morphologic, and immunophenotypic findings suggest that most lymphoid neoplasms with dual translocations of t(14;18) and chromosome 8q24/c-MYC gene rearrangement are high grade with clinical and morphologic features of Burkitt or atypical Burkitt lymphoma/leukemia or diffuse large B-cell lymphoma. With the exception of BCL-2 expression, and <99% of Ki-67 labeling in some cases, the immunophenotype of these neoplasms also fits well with Burkitt or atypical Burkitt lymphoma/leukemia. As affected patients have poor prognosis despite aggressive therapy, knowledge of the presence of both translocations has prognostic value and thus conventional cytogenetic analysis is warranted. For cases without conventional cytogenetic data, FISH analysis is useful in confirming the presence of c-MYC rearrangement in high-grade B-cell lymphoma displaying Burkitt or atypical Burkitt morphology. The presence of BCL-2 expression in a case otherwise resembling Burkitt or atypical Burkitt lymphoma may be a clue for the coexistence of t(14;18). Two cases in this study, due to their morphological features, are truly rare including one case of plasmablastic myeloma and one case of CD10-positive low-grade B-cell lymphoma. We also describe the coexistence of t(14;18) and t(2;8) in a case that morphologically resembled diffuse large B-cell lymphoma.
References
Diebold J, Jaffe ES, Raphael M, et al. Burkitt lymphoma. In: Jaffe ES, Harris NL, Stein H and Vardiman JW (eds). World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Hematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2001, pp 181–184.
Vega F, Medeiros LJ . Chromosomal translocations involved in non-Hodgkin lymphomas. Arch Pathol Lab Med 2003;127:1148–1160.
Hecht JL, Aster JC . Molecular biology of Burkitt's lymphoma. J Clin Oncol 2000;18:3707–3721.
Boxer LM, Dang CV . Translocations involving c-myc and c-myc function. Oncogene 2001;20:5595–5610.
Au WY, Horsman DE, Gascoyne RD, et al. The spectrum of lymphoma with 8q24 aberrations: a clinical, pathological and cytogenetic study of 87 consecutive cases. Leuk Lymph 2004;45:519–528.
Voorhees PM, Carder KA, Smith SV, et al. Follicular lymphoma with a Burkitt translocation—predictor of an aggressive clinical course: a case report and review of the literature. Arch Pathol Lab Med 2004;128:210–213.
Mukhopadhyay S, Readling J, Cotter PD, et al. Transformation of follicular lymphoma to Burkitt-like lymphoma within a single lymph node. Hum Pathol 2005;36:571–575.
Merchant S, Schlette E, Sanger W, et al. Mature B-cell leukemia with more than 55% prolymphocytes: report of 2 cases with Burkitt lymphoma-type chromosomal translocations involving c-MYC. Arch Pathol Lab Med 2003;127:305–309.
Hao S, Sanger W, Onciu M, et al. Mantle cell lymphoma with 8q24 chromosomal abnormalities: a report of 5 cases with blastoid features. Mod Pathol 2002;15:1266–1272.
Lin P, Bueso-Ramos C, Wilson CS, et al. Waldenstrom macroglobulinemia involving extramedullary sites: morphologic and immunophenotypic findings in 44 patients. Am J Surg Pathol 2003;27:1104–1113.
Mitelman F (ed). An International System for Human Cytogenetic Nomenclature. Karger, ISCN: Basel, Switzerland, 1995.
Macpherson N, Lesack D, Klasa R, et al. Small noncleaved, non-Burkitt's (Burkitt-like) lymphoma: cytogenetics predict outcome and reflect clinical presentation. J Clin Oncol 1999;17:1558–1567.
Thangavelu M, Olopade O, Beckman E, et al. Clinical, morphologic, and cytogenetic characteristics of patients with lymphoid malignancies characterized by both t(14;18)(q32;q21) and t(8;14)(q24;q32) or t(8;22)(q24;q11). Genes Chromosomes Cancer 1990;2:147–158.
Dunphy CH, van Deventer HW, Carder KJ, et al. Mature B-cell acute lymphoblastic leukemia with associated translocations (14;18)(q32;q21) and (8;9)(q24;p13). A Burkitt variant? Arch Pathol Lab Med 2003;127:610–613.
Gluck WL, Bigner SH, Borowitz MJ, et al. Acute lymphoblastic leukemia of Burkitt's type (L3 ALL) with 8;22 and 14;18 translocations and absent surface immunoglobulins. Am J Clin Pathol 1986;85:636–640.
Karsan A, Gascoyne RD, Coupland RW, et al. Combination of t(14;18) and a Burkitt's type translocation in B-cell malignancies. Leuk Lymph 1993;10:433–441.
Brito-Babapulle V, Crawford A, Khokhar T, et al. Translocations t(14;18) and t(8;14) with rearranged bcl-2 and c-MYC in a case presenting as B-ALL (L3). Leukemia 1991;5:83–87.
Mufti GJ, Hamblin TJ, Oscier DG, et al. Common ALL with pre-B-cell features showing (8;14) and (14;18) chromosome translocations. Blood 1983;62:1142–1146.
Geisler C, Philip P, Plesner T, et al. Simultaneous presence of translocations t(14;18) and t(2;8) in a case of chronic lymphocytic leukemia. Cancer Genet Cytogenet 1986;22:35–44.
Avet-Loiseau H, Gerson F, Magrangeas F, et al. Rearrangements of the c-MYC oncogene are present in 15% of primary human multiple myeloma tumors. Blood 2001;98:3082–308621.
Vega F, Chang CC, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol 2005;18:806–815.
Marin MC, Hsu B, Stephens LC, et al. The functional basis of c-MYC and bcl-2 complementation during multistep lymphomagenesis in vivo. Exp Cell Res 1995;217:240–247.
McDonnell TJ, Korsmeyer SJ . Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature 1991;349:254–256.
Gauwerky CE, Hoxie J, Nowell PC, et al. Pre B-cell leukemia with a t(8;14) and a t(14;18) translocation is preceded by follicular lymphoma. Oncogene 1998;2:431–435.
Martin-Subero JI, Odero MD, Hernandez R, et al. Amplification of IGH/MYC fusion in clinically aggressive IGH/BCL2-positive germinal center B-cell lymphomas. Genes Chromosomes Cancer 2005;43:414–423.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanungo, A., Medeiros, L., Abruzzo, L. et al. Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol 19, 25–33 (2006). https://doi.org/10.1038/modpathol.3800500
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800500
Keywords
This article is cited by
-
Optimization of high-dose methotrexate prophylaxis for central nervous system relapse in diffuse large B-cell lymphoma: a multicenter analysis
Annals of Hematology (2022)
-
Clinicopathological and genomic analysis of double-hit follicular lymphoma: comparison with high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements
Modern Pathology (2018)
-
De novo acute lymphoblastic leukemia-like disease of high grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a case report and literature review
BMC Clinical Pathology (2017)
-
Prognostic implications of abnormalities of chromosome 13 and the presence of multiple cytogenetic high-risk abnormalities in newly diagnosed multiple myeloma
Blood Cancer Journal (2017)
-
Double-Hit Large B Cell Lymphoma
Current Oncology Reports (2017)