Abstract
Recent data have suggested considerable molecular differences in cancers from various ethnical groups. As molecular features are increasingly used for predicting cancer prognosis and response to therapy, better knowledge of ethnic molecular features is important. To identify potential molecular differences between breast cancers in Europe and the Middle East, we analyzed consecutive breast cancer series from Switzerland (n=2197) and Saudi Arabia (n=204). Tissue microarrays were analyzed by fluorescence in situ hybridization for HER2, CCND1, MYC, and EGFR amplification. The data revealed marked differences between Saudi and Swiss patients. Saudi breast cancers had a markedly higher frequency of HER2 (31 vs 17%; P<0.0001) and MYC (16 vs 5%; P<0.0001) amplifications than Swiss breast cancers. Remarkably, this was partly due to a much higher incidence of grade 3 cancers in the Saudi than in the Swiss population (65 vs 32%; P<0.0001). However, differences in amplification frequency hold also true within grade 3 cancers (HER2: 40 vs 30%, P<0.05; MYC: 22 vs 11%, P=0.002). Interestingly, in combination with known age standardized incidence rates of breast cancer in Saudi Arabia (21.6/100 000) and Switzerland (70.1/100 000), these data suggest that the incidence of high-grade breast cancer is comparable for Saudi and Swiss women, while the incidence of low-grade breast cancers is about 14 times lower in Saudi than for Swiss women. These observations suggest that a difference in genetic susceptibility and/or lifestyle between Saudi and Swiss women has a substantial and much higher than expected impact on the risk of low-grade breast cancer.
Similar content being viewed by others
Main
Breast cancer is one of the most important malignancies of women in all countries. Molecular information is increasingly utilized to select the optimal therapy for individual patients. Already now, analyses of estrogen and progesterone receptors as well as HER2 amplification/overexpression are routine procedures for breast cancer patient management. Recently, DNA array experiments have suggested that gene expression profiles may be better predictors of prognosis and response to therapy than traditional histologic parameters.1, 2, 3 The number of diagnostic molecular analyses for breast cancer and other tumors is expected to increase in the future.
The vast majority of genetic information on breast cancer and other tumors is derived from studying European and US patients. However, increasing evidence suggests the possibility of relevant molecular differences between cancers from patients of different ethnic groups. Differences in the patterns of p53 mutations in breast cancers were reported from Midwest US Caucasian, African-American, Austrian, and Japanese women.4, 5 It was also observed that the frequency and the pattern of germline mutations in BRCA1 and BRCA2 varied between ethnic groups.6 Allelic length of EGFR intron 1 being important for regulating EGFR expression is differing between Asian, Caucasian, and African-American breast cancer patients.7 Most recently, it was suggested that the frequency of EGFR exon 18–21 mutations, predictive for response to Gefitinib (Iressa®), varies considerably between Japanese and US lung cancer patients.8, 9
The aim of this study was to identify potential differences between breast cancers from Western countries and the Middle East. Consecutive series of breast cancers from Switzerland and Saudi Arabia were available for this purpose. In addition to morphologic evaluation, alterations at HER2, MYC, CCND1, and EGFR were selected for this purpose because these oncogenes are often activated due to gene amplification. Gene amplification can be reliably measured independent of potential fixation differences by fluorescence in situ hybridization (FISH). The analysis of two consecutive breast cancer series from Switzerland and Saudi Arabia in a tissue microarray (TMA) format suggests marked biological differences between Arabian and Swiss breast tumors.
Materials and methods
Breast Cancer TMAs
Two sets of TMAs made from formalin-fixed (buffered neutral aqueous 4% solution) breast cancers were used. Both sets were from consecutive series of breast cancers. The first set consisted of 2197 primary breast cancers collected from 1985 to 1999 at the Institute of Pathology, University Hospital Basel, the Institute of Clinical Pathology in Basel, and the Triemli Hospital in Zürich. The median age of these patients was 62 years (26–101 years). The second TMA consisted of samples from all 204 breast cancers from Saudi patients collected at the Pathology Department, Saudi Aramco Hospital from 1988 until 2002. All cancers were first diagnosed at the Aramco hospital (no referrals from outside). The use of the tissue and the data for this project was permitted by the ethical committees of the cantonal hospital of Basel and the King Faisal Cancer Center. The Swiss breast cancers were reviewed by two pathologists (GS, JT) to define the histologic grade according to Elston and Ellis (BRE)10 and the histologic tumor type. The concordance rate of these pathologists was >90% for BRE grading. One of them (GS) also reviewed all Arabian cancers. The median age of Arabian patients was 47 years (28–85 years). TMA construction was as described.11 Briefly, a hematoxylin and eosin (H&E)-stained section was made from each block to define representative tumor regions. Tissue cylinders with a diameter of 0.6-mm were then punched from tumor areas of each ‘donor’ tissue block and brought into six different recipient paraffin blocks each containing between 204 and 522 individual samples using a home made semiautomated tissue arrayer. Sections of 4 μm thickness of the resulting TMAs were transferred to an adhesive-coated slide system (Instrumedics Inc., Hackensack, NJ, USA). An overview of an H&E-stained breast cancer TMA section is shown in Figure 1.
Overview of an H&E-stained breast cancer TMA section (a). Examples of HER2 nonamplified (b) and amplified breast tumors (c). The tumor in panel b contains one to four centromere 17 and one to three HER2 gene signals per cell. The cancer cells in panel c show two centromere 17 and clusters of HER2 gene signals in cancer nuclei.
Fluorescence In Situ Hybridization (FISH)
TMA sections were used for dual labeling FISH. For proteolytic slide pretreatment a commercial kit was utilized (paraffin pretreatment reagent kit, Vysis, Downers Grove, IL, USA). Spectrum-Orange-labeled gene-specific probes were used together with Spectrum-Green-labeled probes for the respective centromere as a reference. The probe combinations were: HER2/centromere 17 (Path Vysion; Vysis), EGFR/centromere 7 (Vysis), CCND1/centromere 11(Vysis), and MYC/centromere 8 (Vysis). Before hybridization, TMA sections were deparaffinized, air dried and dehydrated in 70, 85 and 100% ethanol followed by denaturation for 5 min at 74°C in 70% formamide–2 × SSC solution. After overnight hybridization at 37°C in humidified chamber, slides were washed and counterstained with 0.2 μM DAPI in an antifade solution. For each tumor, the average gene and centromere copy numbers were estimated by one experienced technician (HN). A tumor was considered amplified if the ratio of gene specific signals/centromere signals was ≥2.0. Data from our laboratory have previously shown that diagnosis of amplification based on signal number estimation is highly reliable.12
Statistics
Contingency table analysis and χ2 tests were used to study the relationship between FISH results and morphological parameters.
Results
Histology
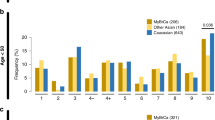
Morphologic and clinical features of Arabian and European breast cancers are summarized in Table 1. The most prominent difference between Swiss and Saudi cancers was a markedly higher BRE grade in Saudi as compared to Swiss cancers. Grade 3 was diagnosed in 65% of Saudi cancers but only 32% of Swiss cancers (P<0.0001). Grade 1 cancers were almost absent in Saudi tumors (6%). Striking differences in the grade distribution were also found in comparisons of tumors of identical diameter or age groups and are shown in Table 2. In addition, there were fewer lobular cancers in the Arabian population (4%) than in the European set (14%; P<0.0001). Grade-specific incidence rates of Saudi and Swiss cancers were calculated based on published breast cancer incidences (Swiss: 70.1/100 000/year; Saudi: 21.6/100 000/year (http://www-dep.iarc.fr/globocan/globocan.html)) and the grade distribution found in this study (Figure 2). This model suggests an only mildly reduced risk of Saudi women to develop high-grade breast cancer (33% less) but an about 14-fold lower risk of grade 1 cancer as compared to Swiss women.
FISH: Technical Aspects
The number of interpretable cases varied only slightly between the different FISH probes and between the two breast cancer sets. In the Swiss set, the fractions of noninformative cases were 26% for HER2, 19% for CCND1, 32% for MYC, and 18% for EGFR. In the Saudi set, the noninformative fractions were 26% for HER2, 18% for CCND1, 25% for MYC, and 55% for EGFR. Reasons for noninformative results were lack of tissue on the TMA, absence of unequivocal tumor cells in the arrayed tissue, or insufficient hybridization. All TMA sections were only hybridized once. No attempts were made to increase the number of interpretable cases by additional experiments since the absolute number of interpretable tumors was sufficient for the purpose of this study. The high rate of noninformative EGFR analyses in the Saudi subset did not prompt further experiments because the number of interpretable cases was sufficient to see that the (small) amplification frequency would be unlikely to vary significantly between Saudi and Swiss patients. Examples of amplified and nonamplified tumors are shown in Figure 1.
FISH: Amplification
Marked differences were seen between Saudi and Swiss patients for two of four gene amplifications (Figure 3). The overall frequencies of HER2 (31 vs 17%; P<0.0001) and MYC (16 vs 5%; P<0.0001) amplification were significantly more frequent in Saudi than in Swiss tumors. As these amplifications are strongly linked to the BRE grade, much of the molecular differences can be explained by the morphologic differences between Saudi and European cancers. However, separate analyses of tumors of identical grade or stage still showed higher amplification frequencies in the Saudi group (Table 3). The differences for HER2 (P<0.05) and MYC (P=0.002) still reached statistical significance within grade 3 cancers. In contrast to MYC and HER2, CCND1 amplifications were less frequent in Arabian than in Swiss cancers (16 vs 20%, P>0.05). The frequency of EGFR amplifications was too low to identify statistically significant differences between the groups.
Discussion
Every effort was taken to avoid patient selection or methodological bias in this project. Consecutive breast cancers were collected in a nonspecialized hospital in Saudi Arabia. Exclusion of tumors with missing clinical follow-up data was the only permitted patient selection for Swiss cancers. This selection has apparently not affected the composition of the Swiss tumor set, as the distribution of BRE grades10 and amplification frequencies12, 13 was in the range of previous studies. To exclude any possible tissue handling bias, FISH was used for molecular analyses only. Insufficient hybridization is always recognized in FISH due to the absence of signals, if suboptimal tissue processing interferes with the hybridization. False negative or false positive FISH results can therefore not occur.
This study reveals fundamental differences between Saudi and Swiss breast cancers. Saudi cancers are mostly high grade and have more than twice as often HER2 and MYC amplifications than Swiss breast cancers. These differences cannot be explained by a lack of a successful early breast cancer detection program or a lower tendency of Saudi women seeing a doctor for breast examination. Studies have shown that grade progression, if at all, occurs only rarely in breast cancer.14 Also, the striking grade differences between Saudi and Swiss cancers are retained in tumors of identical diameter or age groups (Table 2). Grade differences between Saudi and Swiss patients cannot be explained by grading subjectivity. The same pathologist who had already classified more than half of the Swiss cancers reviewed all Saudi tumors. In addition, the substantial molecular differences between Saudi and Swiss breast cancers provide conclusive proof for true differences of the two cancer sets.
Molecular studies have suggested that two pathways exist for breast cancer development, one leading to high-grade tumors and the other to low-grade tumors.15, 16 The predominance of high-grade breast cancers in Saudi Arabia can either be caused by Saudi women having an increased risk of high grade or a reduced risk of low-grade cancers. A calculation of grade-specific breast cancer incidences for Switzerland and Saudi Arabia, which is based on the grade distributions and the published breast cancer incidence in Saudi Arabia (21.6/100 000 women/year) and Switzerland (70.1/100 000 women/year), suggests a massive under-representation of low grade tumors in Saudi Arabia. In fact, Saudi Arabian women appear to have an almost 14-fold lower risk to develop low-grade breast cancer than Swiss women, while their risk to develop grade 3 breast cancer is slightly higher.
The almost complete absence of low-grade breast cancer in Saudi women could best be explained by a striking and in its importance previously unsuspected impact of genetic factors or lifestyle differences on the development of low-grade breast cancer. There is possible support for both hypotheses. Lifestyle differences are significant between Saudi and Swiss women including factors known or suspected to impact the breast cancer risk. For example, Arabian women have a higher frequency of pregnancies, a younger age at first pregnancy, less use of ovulation inhibitors, less tobacco abuse, and no alcohol. Also there may be differences in the diet habits. The pattern of diet habit in Saudi population has changed in recent decades, characterized by increased consumption of high saturated fat.17 Substantial differences in the genetic breast cancer susceptibility would also be conceivable between our patient groups. The Arabian population is genetically unique. A tribal lifestyle with very large families and a substantial amount of consanguinity in these tribes could lead to a high density of a certain genetic make-up within one tribe.18 At least in subpopulations (tribe) this could lead to a massive increase or decrease of inherited traits potentially even in case of polygenic inheritance.
Previous studies using cDNA arrays for breast cancer classification suggested four to five distinct molecular subtypes, one of them is being characterized by HER2 overexpression.19 The observed over-representation of HER2 amplification, even among grade 3 cancers, in the Saudi population would be consistent with an increased risk of Saudi women to develop this particular subtype. If this was true, it would appear that Saudi women not only have a much lower risk of low grade cancers but also a different distribution of molecular subtypes within their high-grade tumors as compared to Swiss women. Ethnical differences involving the HER2 gene have recently been described. Lower frequencies of a single-nucleotide polymorphism (Ile655Val) in the HER2 gene corresponded with a lower incidence and lower risk of breast cancer in continental African populations compared with Saudi, Chinese, Filipino, Caucasian, and African-American women.20 Interestingly, Choi et al21 recently reported a markedly elevated HER2 amplification frequency in Korean breast cancers, similar to Saudi women.
Independent of the reasons for ethnical differences, our data have practical implications. First, treatment with Trastuzumab (Herceptin®) may be considered for a much higher proportion of Saudi (and perhaps Korean) breast cancer patients than for Europeans. As the results of combined Herceptin® therapy of advanced breast cancers are increasingly encouraging,22 ensuring high-quality HER2 testing of breast cancers will be particularly important in these geographic regions. Secondly, it must be considered that different average molecular tumor features in non-Western may also result in a different average response to non-gene-specific cancer therapies than expected from the published US/European data. It cannot be excluded that diagnostic tests based on expression profiling that were able to predict both prognosis and response to cytotoxic therapy in Western breast cancers1, 2, 3 will fail in tumors from other ethnic groups.
In summary, our data show marked differences between Saudi and Swiss breast cancers with an almost complete absence of low-grade cancers in Saudi Arabia. Ethnic variability in genetic cancer subtype distribution may be more common than previously anticipated and may involve also other tumor types than breast cancer. More studies in this field are needed as ethnic variability of cancer biology may affect the general applicability of the products of modern molecular medicine.
References
van't Veer LJ, Dai H, van de Vijver MJ, et al. Expression profiling predicts outcome in breast cancer. Breast Cancer Res 2003;5:57–58.
van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
Huang E, Cheng SH, Dressman H, et al. Gene expression predictors of breast cancer outcomes. Lancet 2003;361:1590–1596.
Hartmann A, Blaszyk H, Saitoh S, et al. High frequency of p53 gene mutations in primary breast cancers in Japanese women, a low-incidence population. Br J Cancer 1996;73:896–901.
Hartmann A, Rosanelli G, Blaszyk H, et al. Novel pattern of P53 mutation in breast cancers from Austrian women. J Clin Invest 1995;95:686–689.
Liede A, Narod SA . Hereditary breast and ovarian cancer in Asia: genetic epidemiology of BRCA1 and BRCA2. Hum Mutat 2002;20:413–424.
Liu W, Innocenti F, Chen P, et al. Interethnic difference in the allelic distribution of human epidermal growth factor receptor intron 1 polymorphism. Clin Cancer Res 2003;9:1009–1012.
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139.
Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500.
Elston CW, Ellis IO . Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19: 403–410.
Sauter G, Simon R, Hillan K . Tissue microarrays in drug discovery. Nat Rev Drug Discov 2003;2:962–972.
Simon R, Nocito A, Hubscher T, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst 2001;93: 1141–1146.
Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 1999;5:1966–1975.
Millis RR, Barnes DM, Lampejo OT, et al. Tumour grade does not change between primary and recurrent mammary carcinoma. Eur J Cancer 1998;34: 548–553.
Menard S, Casalini P, Tomasic G, et al. Pathobiologic identification of two distinct breast carcinoma subsets with diverging clinical behaviors. Breast Cancer Res Treat 1999;55:169–177.
Roylance R, Gorman P, Hanby A, et al. Allelic imbalance analysis of chromosome 16q shows that grade I and grade III invasive ductal breast cancers follow different genetic pathways. J Pathol 2002;196: 32–36.
Hanash KA, Al-Othaimeen A, Kattan S, et al. Prostatic carcinoma: a nutritional disease? Conflicting data from the Kingdom of Saudi Arabia. J Urol 2000;164: 1570–1572.
Becker SM, Al Halees Z, Molina C, et al. Consanguinity and congenital heart disease in Saudi Arabia. Am J Med Genet 2001;99:8–13.
Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–10874.
Ameyaw MM, Tayeb M, Thornton N, et al. Ethnic variation in the HER-2 codon 655 genetic polymorphism previously associated with breast cancer. J Hum Genet 2002;47:172–175.
Choi DH, Shin DB, Lee MH, et al. A comparison of five immunohistochemical biomarkers and HER-2/neu gene amplification by fluorescence in situ hybridization in white and Korean patients with early-onset breast carcinoma. Cancer 2003;98:1587–1595.
Vogel CL, Franco SX . Clinical experience with trastuzumab (herceptin). Breast J 2003;9:452–462.
Acknowledgements
This work was funded by the Schweizerischen Arbeitsgemeinschaft für klinische Krebsforschung (SAKK) (to C Tapia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Kuraya, K., Schraml, P., Sheikh, S. et al. Predominance of high-grade pathway in breast cancer development of Middle East women. Mod Pathol 18, 891–897 (2005). https://doi.org/10.1038/modpathol.3800408
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800408
Keywords
This article is cited by
-
Frequency and clinical characteristics of HER2 over-expressed breast cancer in Saudi Arabia: a retrospective study
BMC Women's Health (2021)
-
Presentation of breast cancer, help seeking behaviour and experience of patients in their cancer journey in Singapore: a qualitative study
BMC Cancer (2020)
-
High prevalence of deleterious BRCA1 and BRCA2 germline mutations in arab breast and ovarian cancer patients
Breast Cancer Research and Treatment (2018)
-
XIAP over-expression is an independent poor prognostic marker in Middle Eastern breast cancer and can be targeted to induce efficient apoptosis
BMC Cancer (2017)
-
Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; hospitals based case series
BMC Cancer (2017)