Abstract
Gonadoblastoma is an unusual mixed germ cell–sex cord–stromal tumor that has the potential for malignant transformation and 30% of all patients with gonadoblastoma develop germ cell tumors mainly dysgerminoma/seminoma. An additional 10% gives rise to other malignant germ cell neoplasms. This tumor affects a subset of patients with intersex disorders. The age at diagnosis is variable ranging from birth to the fourth decade, but around 94% of cases are diagnosed during the first three decades of life and there are few cases with gonadoblastoma diagnosed in infants. In this paper, we present the histological and molecular findings of four patients with gonadal dysgenesis who developed gonadoblastoma in the first 2 years of life and one case with bilateral dysgerminoma diagnosed at 15 years of age. The sex chromosomes of mosaic patients do not distribute homogenously in dysgenetic gonads; however, statistical analysis of FISH results revealed significant differences between the XY cell line in the gonadoblastoma compared with the dysgenetic testis. Our cases demonstrate that tumors could be present at a very early age, so the prophylactic removal of the gonads is advised.
Similar content being viewed by others
Main
Gonadoblastoma is an unusual mixed germ cell–sex cord–stromal tumor that has the potential for malignant transformation. This unique gonadal neoplasm was described by Scully1 in 1957 as a benign tumor that affects mostly a subset of patients with intersex disorders. The syndromes associated with a clear risk for tumor development are mixed gonadal dysgenesis,1, 2, 3 some patients with Turner phenotype,4 occasionally in 46,XY male pseudohermaphroditism2, 3, 4, 5 and there is also a reported case in a 46,XX/46,XY true hermaphrodite.6 Tumor development in these patients is associated with the presence of either normal or abnormal Y-chromosomes or molecular evidence for Y-derived sequences and intrabdominal location of the abnormal gonad.3, 7, 8 Histologically, the tumor is composed of well-circumscribed round to oval nests with a mixture of germ cells and sex-cord-type cells resembling immature Sertoli or granulosa cells that often show central calcification.9 Gonadoblastoma per se does not show invasive behavior but 30% of the specimens demonstrate evidence of overgrowth by the germinal component. In this case, the lesion is no longer considered benign and it is termed dysgerminoma/seminoma. An additional 10% of gonadoblastomas develops different types of germ cell neoplasias, such as yolk sac tumor, immature teratoma, embryonal carcinoma and choriocarcinoma.9 Around 94% of cases are diagnosed during the first three decades; however, the age at diagnosis is variable ranging from birth up to the fourth decade of life. Diagnosis in infants is rare;10, 11, 12 however, several cases were diagnosed around 10 year of age.13, 14, 15, 16, 17 The presence of gonadoblastoma in young patients and the potential risk for malignant transformation is the main reason for early diagnosis and treatment in some cases of intersex patients. In this paper, we present the histological and molecular findings of four patients with gonadal dysgenesis, who developed gonadoblastoma in the first 2 years of life and one case with bilateral dysgerminoma diagnosed at 15 years of age.
Materials and methods
We studied five patients with mixed gonadal dysgenesis, who developed gonadoblastoma at an early age. All cases were of Mexican mestizo origin and consulted because of ambiguous genitalia. Clinical evaluation in cases 1–4 was performed between 1 and 2 years of age while the fifth patient was evaluated at 15 years old. All cases were sporadic, family history was negative for genital ambiguity, genetic diseases, neoplasia and consanguinity. Clinical features, age of diagnosis, sex of rearing, karyotype and gonadal findings are summarized in Table 1.
GTG chromosomal analysis was performed on peripheral blood leukocytes analyzing at least 100 metaphases in each case. The endocrinal profile includes LH, FSH, ACTH, testosterone/dihidrotestosterone (after GCH stimulation), 17-OH progesterone and cortisol.
Histological Analysis
Hematoxylin and eosin sections of all gonads were reviewed by one of the authors.
PCR Analysis
In patient 1, PCR in DNA obtained from leukocytes, analyzing different regions of Y-chromosome (PABY, ZFY, SRY, Ycen and Yqh) was performed according to a previous report.18 An X-chromosome alphoid centromeric repeat was also amplified as an internal positive control. The total PCR volume was 25 μl; all amplifications included 50 ng of DNA, 100 ng of each primer, 1 U of Taq polymerase, 1.5 mM MgCl2, and 80 μM of each dNTP. Program for SRY amplification was: 94°C (5 min), 94°C (1 min), 68°C (1 min) and 72°C (2 min) for 35 cycles and then 72°C (10 min). All assays were performed in triplicate including a blank as negative control and DNA from a normal male as a positive control.
FISH Analysis
FISH analysis was carried out in paraffin-embedded gonadal tissue sections. Using an X and Y centromeric region labeled with spectrum green and orange, respectively; as an internal control we used a centromeric probe of chromosome 18 labeled in aqua. Hybridization conditions were previously described by Pinkel et al.19, 20 After hybridization, the number of X- and Y-chromosome signals per nucleus in the tumor and in the dysgenetic testis was scored by two observers, 300 nuclei were counted from each tissue, the tumor and the tissue score were performed independent and repeated two times. Only nuclei where two 18 centromeres were scored and all damage, overlapping or without hybridization signal nuclei were discarded. The hybridization efficiency, defined as the percentage of intact nonoverlapping cells containing signal was 96%. Statistical analysis of the FISH data was performed using a χ2 test.
Results
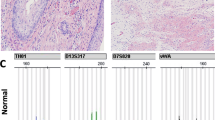
Patient 1. At the age 1 month this phenotypic female assigned with stigmata of Turner and 45,X karyotype underwent laparoscopy because of hyperandrogenic status (basal testosterone levels 280 ng/ml; reference value 3–10 ng/ml). A right ‘paraovarian cyst’ measuring approximately 1 cm was observed and excised. Histological analyses revealed a juvenile granulosa cell tumor (JGCT) (Figure 1d and e). The diagnosis was confirmed by immunoperoxidase stains that were positive for cytokeratin, protein S-100, vimentin and inhibin. The child was small for age and on the following months she had failure to thrive. A second laparoscopic surgery was performed and both gonads and internal genitalia were removed. There was a dysgenetic testis measuring 1.0 cm in diameter with focal gonadoblastoma, spermatic cord and fallopian tube on the right side while a streak measuring 0.8 × 0.4 × 0.2 cm with fallopian tube was on the left side. A central uterus was also removed (Figure 1a–c).
Patient 1: (a) Streak gonad, (b) uterus, bilateral tubes, streak on the left side and dysgenetic testis with gonadoblastoma on the right side, (c) gonadoblastoma, (d) juvenile granulosa cell tumor (JGCT), (e) immunoperoxidase for inhibin on JGCT, (f) dysgerminoma in patient 5. FISH analysis using an X centromere probe (green), a Y centromere probe (red) and an 18 centromeric probe (aqua). Patient 1: (g) left streak showing a very low-level of Y hybridization signal and (h) right gonadoblastoma, showing a higher level of Y signal than the streak. Patient 3: (i) FISH analysis in the right side gonadoblastoma exhibiting a higher hybridization signal for the Y-chromosome.
Patient 2. Laparoscopic findings at 11 months of age in this male assigned infant revealed bilateral gonads resembling ovaries, fallopian duct-like structures and a central uterus. A biopsy taken from both gonads showed bilateral gonadoblastoma.
Patient 3. A laparotomy was performed in this 2-month-old male assigned that revealed a 1.2 cm in diameter left inguinal testis that was resected, histologically it was diagnosed as a dysgenetic testis. A second surgery was performed at age 24 months, an uterus and the right gonad measuring 1.5 × 0.5 × 0.5 cm were removed; histologically this gonad was replaced by gonadoblastoma.
Patient 4. Abdominal gonads were removed through laparotomy at 28 months old in a female reared patient. The right gonad was a 2.5 cm in diameter dysgenetic testis with peripheral gonadoblastoma. Towards the hilus a small streak was observed as well as a rudimentary epididymis and fallopian tube. On the left side only a fallopian tube was identified.
Patient 5. A laparotomy was performed due to an abdominal mass at 15 years of age. A right side tumor measuring 6.0 cm in largest dimension was diagnosed as dysgerminoma; it had areas of calcification suggesting a ‘burned-out gonadoblastoma’ (Figure 1f). The left gonad measured 4.5 cm in its major dimension and histologically it was replaced by gonadoblastoma with focal transformation to dysgerminoma. The nature of the gonads could not be elucidated.
PCR and FISH Analysis
PCR amplification of DNA obtained from leukocytes was performed in patient 1 where all Y sequences analyzed were present disclosing a Y-positive cell line, therefore, the final karyotype was 45,X/46,XY (data not shown).
FISH analysis was performed to search a hidden mosaicism for X monosomy, as well as to know the X- and Y-chromosome cell distribution in the gonadal tissue and in the tumor on these patients. Results are summarized in Table 2. In patient 1, a low percentage of Y hybridization signal was observed compared with the 45,X cell line in the left side gonadoblastoma and in the right side streak (Figure 1g, h). These data confirm the presence of an XY cell line in this patient. However, the gonadoblastoma contained three more times the XY cells than the streak but the low level of Y-bearing cells observed diminished the significance of these findings. In case 3, we scored 300 cells and the results revealed that the 46,XY cell line was 79% in the gonadoblastoma (Figure 1i), while the presence of XY cells in the dysgenetic testis was 46%. These results indicated that the Y-bearing cells are 1.7 times more frequent in the tumor than in the testis (P<0.001). The hybridization efficiency was 96% in the tumor and in the dysgenetic testis, thus, the data obtained cannot be attributed to technical differences. In patient 2, the GTG karyotype in peripheral blood was 46,XY. In order to identify the presence of a hidden 45,X cell line in the gonad, we performed FISH analysis in the bilateral tumors. The results showed the presence of some cells with only an X hybridization signal, confirming the presence of a hidden X monosomy and modifying the original karyotype to 45,X/46,XY. In patient 5, the analysis of the bilateral gonadoblastoma/dysgerminoma revealed that approximately 60% of the cells were XY in the right side, while the analysis of the contralateral tumor showed that 65% of the cells were XY and the rest had only one X.
Discussion
Pure gonadoblastoma is regarded as an in situ form of germ cell tumor; it does not behave as a malignant lesion but approximately 30% of all patients with gonadoblastoma develop germ cell tumors mainly dysgerminoma/seminoma.9 An additional 10% gives rise to other malignant germ cell neoplasms such as yolk sac tumor, immature teratoma, embryonal carcinoma or choriocarcinoma. These tumors have a malignant behavior; therefore early prophylactic removal of the gonads in these patients is advised.1, 9 This kind of tumor affects almost exclusively a subset of patients with intersex disorders, however, there are three cases in the literature where gonadoblastoma developed in normal males.21 The well-known syndromes associated with a risk for tumor development are: mixed gonadal dysgenesis, some patients with Turner phenotype and in several cases of 46,XY male pseudohermaphroditism.2, 3, 4, 5, 6, 7 There is also a reported case of this type of tumor in a 46,XX/46,XY true hermaphrodite.6 The tumor development in these patients had been associated with the presence of normal or abnormal Y-chromosomes, molecular evidence for Y-derived sequences and intrabdominal location of the abnormal gonads.3, 4, 7, 8, 18 The age at diagnosis is variable ranging from birth to the fourth decade; around 94% of cases reported in the literature was diagnosed during the second or third decades of life.22 Data in the literature revealed 10 cases where the tumor developed during childhood, five of them had a 45,X/46,XY mixed gonadal dysgenesis and the diagnosis was performed only in one case at 9 months old while the rest were diagnosed around 10 years of age.10, 11, 12, 13, 14, 15 The other five patients had a 46,XY gonadal dysgenesis, one of them, a 3-year-old girl had campomelic dysplasia, sex reversal and bilateral gonadoblastoma while in the other four a WT1 mutation was observed.16, 23, 24, 25, 26 The presence of a suppress tumor mutation in these individuals could participate in early tumor development.
Here we present the histological and FISH analysis in four infants and in one 15-year-old patient with mixed gonadal dysgenesis and gonadal tumor. Four of them were diagnosed during the first 2 years of life and in the fifth case an abdominal mass was the cause of the diagnosis.
Gonadoblastoma is a unique tumor in which germ cells and immature Sertoli/granulose cells are intimately mixed, in addition to the germ cell overgrowth, the stromal-cell component is also capable of neoplastic transformation. In case 1, a small paraovarian cystic tumor proved to be a JGCT. Although rare, this neoplasm has been described in testicles of infants and children with abnormal sexual development27, 28, 29 and in some patients with no apparent sexual abnormalities.12 Histological analysis of the tumors in cases 2–4 revealed typical characteristics of gonadoblastoma. In case 5, a dysgerminoma/seminoma with extensive calcifications was observed in the right side and a gonadoblastoma with overgrowth of germ cells in the left side.
Molecular findings in cases 1 and 2 revealed the presence of hidden mosaicisms. Case 1 was diagnosed by conventional cytogenetics as 45,X but PCR in DNA obtained from peripheral blood and FISH performed in gonadal tissue confirmed the presence of a low-level XY cell line. These findings are in agreement with several reports, in which Y material in Turner Syndrome could be present in approximately 12% of the cases.30, 31 Previous studies suggested that 30% of these patients have the potential risk of developing gonadoblastoma.31, 32 However, new molecular data indicate that the occurrence of gonadoblastoma among the Y-positive Turner patients is lower than 10% and this risk increases with age.4 Case 2 was originally diagnosed as 46,XY, but FISH analysis in gonadal tissue showed a 45,X cell line. Recently, hidden mosaicisms for X- and Y-chromosomes were detected by FISH analysis and associated with true hermaphroditism.33 The presence of a hidden X-bearing cell line in case 2 confirms that this mechanism could also participate in the pathogenesis of the abnormal gonad and we propose to perform molecular analysis in all the dysgenetic gonads. We also analyzed the differences in cell distribution between the gonads and the tumors in cases 1, 3 and 5. A higher proportion of XY cells were observed within the tumors compared with the dysgenetic gonads (Table 2). Our data obtained by FISH analysis demonstrated that the sex chromosomes of mosaic patients do not distribute homogenously in dysgenetic gonads as was previously described3 and confirms the original findings reported by Iezzoni et al34 where the proportion of the XY cell line was higher in the tumor than in the stromal cells. These authors proposed that the presence of Y-chromosome material participates in the pathogenesis of the tumor. Our data are in agreement with these findings and suggest that the previously proposed TPSY gene (testis-specific protein, Y encoded) localized within the GBY locus (gonadoblastoma locus on the Y-chromosome), participates in the multistep malignant transformation.35, 36, 37 The higher proportion of the Y cell line observed in the tumor suggests a TSPY overexpression. Recently, Skaletsky et al38 proposed that TSPY is localized in a Y-chromosome amplicon repeated region. This cluster is the largest and homogenous tandem array identified in the human genome and these sequences have the potential for gene conversion. The early age of tumor development in these patients could be attributed to gene conversion leading to microrearrangements on the Y-chromosome that upset the TSPY function. Another possibility could be an abnormal relation between the environmental influence and the genome. In patients with pure 46,XY gonadal dysgenesis, the calculated risk for malignancy is 28% by the age of 20 years while for patients with mixed gonadal dysgenesis it is 19% at the same age;22 nevertheless, our cases demonstrate that tumors could be present at a very early age, so prophylactic removal of the gonads is advised.
References
Scully RE . Gonadoblastoma. A review of 74 cases. Cancer 1970;25:1340–1356.
Gourlay WA, Johnson HW, Pantzar JT, et al. Gonadal tumors in disorders of sexual differentiation. Urology 1994;43:537–540.
Chemes H, Muzulin PM, Venara MC, et al. Early manifestations of testicular dysgenesis in children: pathological phenotypes, karyotype correlations and precursor stages of tumors development. APMIS 2003; 111:12–23.
Gravholt CH, Fedder J, Naeraa RW, et al. Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: a population study. J Clin Endocrinol Metab 2000;85:3199–3202.
Chen CP, Chern SR, Wang TY, et al. Androgen receptor gene mutations in 46,XY females with germ cell tumors. Hum Reprod 1999;14:664–670.
Talerman A, Verp MS, Senekjian E, et al. True hermaphrodite with bilateral ovotestes, bilateral gonadoblastomas and dysgerminomas, 46,XX/46,XY karyotype, and successful pregnancy. Cancer 1990;66:2668–2672.
Vlasak I, Plochl E, Kronberger G, et al. Screening of patients with Turner syndrome for ‘hidden’ Y-mosaicism. Klin Padiat 1999;211:30–34.
Slowikowska-Hilczer J, Romer TE, Kula K . Neoplastic potential of germ cells in relation to disturbances of gonadal organogenesis and changes in karyotype. J Androl 2003;24:270–278.
Russell P, Robboy SJ, Anderson MC . Germ cell tumors of the ovaries. In: Robboy SJ, Anderson MC, Russell P (eds). Pathology of the Female Reproductive Tract. Churchill Livingston: London, 2002, pp 641–690.
Dumic M, Jukic S, Batinica S, et al. Bilateral gonadoblastoma in a 9-month-old infant with 46,XY gonadal dysgenesis. J Endocrinol Invest 1993;16:291–293.
Haddad NG, Walvoord EC, Cain MP, et al. Seminoma and a gonadoblastoma in an infant with mixed gonadal dysgenesis. J Pediatr 2003;143:136.
Goswith J, Pettinato G, Manivel JC . Testicular sex cord stromal tumors in children: Clinicopathologic study of sixteen children with review of the literature. Pediatr Pathol Lab Med 1996;16:451–470.
Gibbons B, Tan SY, Yu CC, et al. Risk of gonadoblastoma in female patients with Y chromosome abnormalities and dysgenetic gonads. J Pediatr Child Health 1999;35:210–213.
Obata NH, Nakashima N, Kawai M, et al. Gonadoblastoma with dysgerminoma in one ovary and gonadoblastoma with dysgerminoma and yolk sac tumor in the contralateral ovary in a girl with 46,XX karyotype. Gynecol Oncol 1995;58:124–128.
Krawczynski M, Walczak M, Miskowiak B, et al. Gonocytoma III (gonadoblastoma) in a 12-year-old girl with mixed gonadal dysgenesis. Endokrynol Pol 1976;27:299–306.
Auber F, Lortat-Jacob S, Sarnacki S, et al. Surgical management and genotype/phenotype correlations in WT1 gene-related diseases Drash, Frasier syndromes. J Pediatr Surg 2003;38:124–129.
Iliev DI, Ranke MB, Wollmann HA . Mixed gonadal dysgenesis and precocious puberty. Horm Res 2002;58:30–33.
Lopez M, Canto P, Aguinaga M, et al. Frequency of Y chromosomal material in Mexican patients with Ullrich-Turner syndrome. Am J Med Genet 1998;5: 120–124.
Pinkel D, Gray JW, Trask B . Cytogenetic analysis by in situ hybridization with flourescently labeled nucleic acid probes. Cold Spring Harb Symp Quant Biol 1986;1:151–157.
Pinkel D, Straume T, Gray JW . Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 1986;83:2934–2938.
Hatano T, Yoshino Y, Kawashima Y, et al. Case of gonadoblastoma in a 9-year-old boy without physical abnormalities. Int J Urol 1999;6:164–166.
Scully RE . Tumors of the ovary, mal developed gonads, fallopian tubes and Broad Ligament. In: Scully RE, Young RH, Clement PB (eds). Atlas of Tumor Pathology, 3rd edn. Armed Forces Institute of Pathology: Washington, DC, 1996, pp 307–312.
Hong JR, Barber M, Scott CI, et al. 3-Year-old phenotypic female with campomelic dysplasia and bilateral gonadoblastoma. J Pediatr Surg 1995;30:1735–1737.
Perez de Nanclares G, Castano L, Bilbao JR, et al. Molecular analysis of Frasier syndrome: mutation in the WT1 gene in a girl with gonadal dysgenesis and nephronophthisis. J Pediatr Endocrinol Metab 2002; 15:1047–1050.
Shimoyama H, Nakajima M, Naka H, et al. A girl with bilateral ovarian tumors: Frasier syndrome. Eur J Pediatr 2002;16:81–83.
Manivel J, Sibley R, Dehner L . Complete and incomplete Drash syndrome: a clinicopathologic study of six cases of a dysontogenetic-neoplastic complex. Hum Pathol 1987;18:80–89.
Tanaka Y, Sasaki Y, Tachibana K, et al. Testicular juvenile granulosa cell tumor in an infant with X/XY mosaicism clinically diagnosed as true hermaphroditism. Am J Surg Pathol 1994;18:316–322.
Young RH, Lawrence WD, Scully RD . Juvenile granulosa cell tumor. Another neoplasm associated with abnormal chromosomes and ambiguous genitalia. Am J Surg Pathol 1985;9:737–743.
Raju U, Fine G, Warrier R, et al. Congenital testicular juvenile granulosa cell tumor in a neonate with X/XY mosaicism. Am J Surg Pathol 1986;10:577–583.
Saenger P, Wikland KA, Conway GS, et al. Recommendations for the diagnosis and management of Turner syndrome. J Clin Endocrinol Metab 2001;86: 3061–3069.
Schellhas HF . Malignant potential of the dysgenetic gonad. II. Obstet Gynecol 1974;44:455–462.
Verp MS, Simpson JL . Abnormal sexual differentiation and neoplasia. Cancer Genet Cytogenet 1987;25:191–218.
Queipo G, Zenteno JC, Pena R, et al. Molecular analysis in true hermaphroditism: demonstration of low-level hidden mosaicism for Y-derived sequences in 46,XX cases. Hum Genet 2002;111:278–283.
Iezzoni JC, Von Kap-Herr C, Golden WL, et al. Gonadoblastomas in 45,X/46,XY mosaicism: analysis of Y chromosome distribution by fluorescence in situ hybridization. Am J Clin Pathol 1997;108:197–201.
Lau YF, Lau HW, Komuves LG . Expression pattern of a gonadoblastoma candidate gene suggests a role of the Y chromosome in prostate cancer. Cytogenet Genome Res 2003;101:250–260.
Page DC . Hypothesis: a Y-chromosomal gene causes gonadoblastoma in dysgenetic gonads. Development 1987;101:151–155.
Salo P, Kaariainen H, Petrovic V, et al. Molecular mapping of the putative gonadoblastoma locus on the Y chromosome. Genes Chromosomes Cancer 1995;14: 210–214.
Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003;423: 825–837.
Acknowledgements
This work was supported by a DGAPA Grant number IN206902-3, the Research Division of the Hospital General de México and CONACYT Grant number 45209-M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peña-Alonso, R., Nieto, K., Alvarez, R. et al. Distribution of Y-chromosome-bearing cells in gonadoblastoma and dysgenetic testis in 45,X/46,XY infants. Mod Pathol 18, 439–445 (2005). https://doi.org/10.1038/modpathol.3800293
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800293