Abstract
Lymphatic invasion and nodal metastasis are predictors of shorter disease-free and overall survival in carcinoma of the uterine cervix. The monoclonal antibody D2-40, which reacts with the oncofetal membrane antigen M2A, is a new selective marker for lymphatic endothelium, and has been shown to be useful in identifying the presence of lymphatic invasion in various malignant neoplasms, including cervical carcinoma. However, the reactivity of the tumor cells with D2-40 has not yet been evaluated. In this study, we examined the pattern of D2-40 immunoreactivity in a series of 138 invasive squamous cell carcinomas of the uterine cervix. We correlated the presence and extent of D2-40 immunoreactivity in the tumor cells with various clinicopathologic features, the presence of lymphatic invasion, lymph node metastasis and outcome. Diffuse or focal D2-40 immunoreactivity was present in 17 (12%) and 81 (59%) tumors, respectively, while 40 (29%) tumors showed no immunoreactivity. Lymphatic invasion and nodal metastasis were present in 56 and 29% of tumors, respectively. Tumor emboli within lymphatic spaces and metastatic tumor foci in lymph nodes showed no immunoreactivity in 86 and 80% of the cases, respectively. Lymphatic invasion and nodal metastasis were significantly more common in tumors showing low D2-40 immunoreactivity (P<0.0001 and 0.022, respectively). D2-40 immunoreactivity showed no correlation with any other clinicopathologic features examined, including tumor size, grade and FIGO stage. In univariate analysis low D2-40 immunoreactivity was significantly associated with shorter recurrence-free, but not with overall survival. Our studies suggest that D2-40 immunostaining may serve as a marker for increased risk of lymphatic invasion and tumor recurrence in cervical biopsy material. Further study of the biological function of the M2A antigen may shed some light on the interaction of tumor cells with lymphatics.
Similar content being viewed by others
Main
The role of angiogenesis in the development and progression of solid tumors, including cervical carcinoma, has been established.1, 2, 3, 4 Besides being important for tumor progression, angiogenesis plays a crucial role in the dissemination of tumor cells via the blood stream.5 However, in cervical cancers the earliest feature of disseminated disease is regional lymph node involvement. In addition to International Federation of Obstetrics and Gynecology (FIGO) stage and tumor volume, lymphatic invasion and nodal metastasis have been found to be predictors of shorter disease-free and overall survival in carcinoma of the uterine cervix.6, 7, 8, 9 Despite its major role in tumor dissemination, little is known about the role of tumor lymphangiogenesis in metastasis and whether lymphatic spread occurs via pre-existing lymphatic vessels or lymphatic vessels newly formed by lymphangiogenesis.10, 11
Much of the difficulty in analyzing lymphatics was due to the lack of lymphatic-specific markers suitable for immunohistochemistry on formalin-fixed paraffin-embedded material that could be used to discriminate between lymphatics and blood vessels.12 The recently developed monoclonal antibody D2-40, which reacts with the oncofetal membrane antigen M2A identified in ovarian carcinoma cell lines and germ-cell tumors,13 was reported to be a selective marker for lymphatic endothelium, and has been shown to be useful in identifying the presence of lymphatic invasion in various malignant neoplasms,14 including cervical carcinoma. Although highly expressed in seminomatous germ-cell tumors (12), the reactivity of the tumor cells themselves with the D2-40 antibody has not yet been evaluated in other neoplasms. In the present study, we examined the expression of the M2A antigen in a series of cervical squamous cell carcinomas by immunohistochemistry using the D2-40 antibody.
Materials and methods
A total of 138 cases of cervical biopsies (n=22), cone biopsies (n=2) and radical hysterectomies (n=114) performed for invasive squamous cell carcinoma (ISCC) were selected from the Surgical Pathology files of the University of Pennsylvania Medical Center. Hematoxylin- and eosin (H&E)-stained slides of all cases were reviewed and the diagnoses confirmed. In addition to the invasive carcinoma, carcinoma in situ and benign cervical squamous epithelium were present in the representative tumor sections selected for the study in 64 and 94 cases, respectively. The clinicopathologic features of the tumors are summarized in Table 1. Staging was defined according to the FIGO staging system,15 and this information was available in 127 cases. Tumor grade was determined according to established criteria.16 All tumors were evaluated for the presence of lymphatic space involvement based on all available H&E-stained sections. Cases with only biopsy material available for review were included in the analysis only if lymphatic space involvement was identified in the biopsy material. Pelvic/para-aortic lymph node dissection was performed in 105 (76%) cases; the median number of lymph nodes per case examined was 22 (range 1–82). Lymph node metastasis was present in 30 (29%) cases; the median number of positive lymph nodes was 2 (range 1–7). Primary treatment was surgical in 116 cases, while primary radiation or combined radiation and chemotherapy was administered in 14 and eight cases, respectively. Following primary surgery, 48 patients received adjuvant treatment, consisting of radiation in 40, and combination radiation and chemotherapy in eight cases, respectively. Follow-up of patients was performed on the basis of information reported in the clinical histories. We considered as uncensored only records of patients who died of disease; we considered as censored records of all patients who were alive at follow-up or patients who died of a cause not related to the disease. Study protocols were approved by the University of Pennsylvania Institutional Review Board.
Immunohistochemical assays were performed on formalin-fixed paraffin-embedded sections. Sections (5 μm thick) were cut and deparaffinized in xylene and rehydrated in graded alcohols. Slides were boiled in 1 × EDTA buffer (LabVision, Fremont, CA, USA) for 20 min. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in methanol for 20 min. Slides were incubated with the D2-40 monoclonal antibody (1:25 dilution, Signet Laboratories, Dedham, MA, USA) for 1 h at room temperature. Immunohistochemical staining was performed on a DAKOCytomation Autostainer using the EnVision+HRP DAB system (DAKOCytomation, Carpinteria, CA, USA), according to the manufacturer's recommendations.
D2-40 immunoreactivity in the tumor cells was evaluated semiquantitatively on a four-tiered scale. Cytoplasmic immunoreactivity was considered positive. The percentage of weakly, moderately and strongly staining cells was determined, and a staining score was calculated as follows: score (out of maximum of 300)=sum of 1 × percentage of weak, 2 × percentage of moderate and 3 × percentage of strong staining.17 For the purposes of statistical analysis, tumors were considered to show low or high D2-40 immunoreactivity if the immunostaining score was less or more than the mean score plus 2 standard deviations for benign cervical squamous epithelia (score 25), respectively.
The Wilcoxon signed-rank test was used for the comparison of median D2-40 immunohistochemical staining levels in invasive and in situ carcinomas and adjacent benign squamous epithelia. Median D2-40 immunohistochemical staining levels were compared using the Kruskal–Wallis one-way analysis of variance by ranks followed by Dunn's multiple comparison test, when appropriate. High vs low immunostaining in carcinomas was compared using the χ2-test. Survival curves were plotted using the method of Kaplan and Meier and compared using the log-rank test. A Cox proportional hazards model was used to assess the effect of tumor variables on survival. The end points were the overall survival from the day of surgery. Statistical significance was determined if the two-sided P-value of a test was less than 0.05. Computations were performed using the Graphpad Prizm (Version 4, GraphPad Software, San Diego, CA, USA) and SYSTAT (Version 10.2, SYSTAT Software Inc., Richmond, CA, USA) softwares.
Results
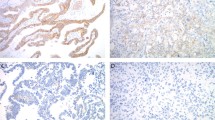
The results of D2-40 immunohistochemical assays are illustrated and summarized in Figure 1. In benign cervical squamous epithelia moderate-to-strong D2-40 immunoreactivity was observed exclusively in the basal cell layer in 93 (99%) of 94 cases (Figure 1a), with a median score of 13.5 (mean±s.e.m.: 12.0±0.5). Moderate-to-strong immunoreactivity, usually confined to the lower third of the epithelium was seen in 59 (92%) of 64 squamous cell carcinomas in situ (median score 30.0, mean±s.e.m.: 44.8±6.0) (Figure 1b). In contrast, invasive squamous cell carcinomas showed one of three patterns of D2-40 immunoreactivity: no immunostaining (score 0) was seen in 40 (29%) of 138 cases (Figure 1c). Focal immunoreactivity present in less than 50% of tumor cells was seen in 81 (59%) cases, with a median score of 25 (mean±s.e.m.: 34.7±3.4). In these cases, D2-40 staining was localized to the periphery of the invading tumor nests and no staining was present in the central, sometimes more differentiated tumor cells, mimicking the pattern seen in benign squamous epithelia (Figure 1d). Diffuse D2-40 immunoreactivity present in at least 50% of the tumor cells was observed in 17 (12%) cases (Figure 1e), with a median score of 180 (mean±s.e.m.: 176.8±10.1). The level of D2-40 reactivity was significantly higher in carcinomas in situ compared to benign cervical squamous epithelia (P<0.001, Kruskal–Wallis test), and to all invasive carcinomas combined (P<0.01, Kruskal–Wallis test) (Figure 1f).
(a) In benign cervical squamous epithelium, D2-40 immunoreactivity is strictly localized to the basal cell layer. Note the lack of immunostaining in the cervical stroma. (b) Strong D2-40 immunostaining in the basal portion of squamous cell carcinoma in situ. The stroma underlying the in situ carcinoma shows moderate immunoreactivity. Invasive squamous cell carcinomas showed three immunostaining patterns: lack of reactivity (c), focal staining usually present at the edge infiltrating tumor nests (d) or diffuse reactivity (e) (immunohistochemical stains for D2-40 with hematoxylin counterstain, original magnification × 100). (f) D2-40 immunoreactivity in benign cervical squamous epithelia, carcinoma in situ and invasive squamous cell carcinomas. Lines indicate median immunostaining score values. All: all invasive tumors; low: invasive tumors showing low D2-40 immunoreactivity; high: invasive tumors showing high D2-40 immunoreactivity. **P<0.01; ***P<0.001 (Kruskal–Wallis test).
In addition to epithelial cells, weak-to-moderate D2-40 reactivity was also seen in the stroma of invasive tumors in 121 (88%) cases (Figures 1b–e). In contrast, in cervical stroma not associated with tumors, weak D2-40 staining was present in only 4 (3%) cases (Figure 1a). The difference in immunoreactivity between ‘benign stroma’ and stroma associated with invasive carcinoma was statistically highly significant (P<0.0001, χ2-test). As expected, D2-40 strongly stained the endothelial cells of lymphatic channels, but did not react with endothelial cells of blood vessels, as determined histologically (Figure 2). D2-40 immunostaining also highlighted the presence of lymphatic invasion, usually present at the periphery of tumors (Figure 2). Tumor emboli present within lymphatic spaces were negative for D2-40 staining in 59 (86%) of 69 cases (Figures 2b and c). Indeed, lymphatic tumor emboli were even negative in 12 of 16 cases where the main tumor mass demonstrated D2-40 immunoreactivity. Metastatic carcinomas within lymph nodes were negative for D2-40 immunoreactivity in 24 (80%) cases, while usually focal weak staining in 5–10% of the tumor cells was seen in the remaining six cases.
(a–c) D2-40 immunostaining highlights lymphatic invasion in cervical squamous cell carcinomas. D2-40 shows strong specific reactivity with lymphatic endothelial cells, while the endothelial cell of blood vessels shows no immunostaining (c). Note that the tumor cell emboli within lymphatic spaces are negative for D2-40 immunoreactivity. (d) Metastatic tumor foci in lymph nodes show no D2-40 immunostaining. (e) Comparison of D2-40 immunoreactivity in invasive cervical squamous cell carcinomas showing absence or presence of lymphatic invasion and lymph node metastasis (Neg LVI, Pos LVI, Neg LN and Pos LN, respectively). Lines indicate median immunostaining score values. *P<0.05; ***P<0.0001 (Mann–Whitney test).
For the purposes of statistical analysis, tumors showing D2-40 immunostaining with a score of less than 25 or 25 and higher were considered to have low (79 cases) or high (59 cases) immunoreactivity, respectively. D2-40 immunoreactivity in invasive cervical squamous cell carcinomas showed no statistically significant correlation with patient age, tumor size, depth of invasion, tumor grade or FIGO stage (Tables 2 and 3). However, tumors associated with the presence of lymphatic invasion and lymph node metastasis showed significantly lower D2-40 immunoreactivity (Table 2). Similarly, when carcinomas showing low and high D2-40 reactivity were compared, lymphatic invasion and lymph node metastases were significantly more common in tumors with low D2-40 immunoreactivity (Table 3).
During the follow-up interval, tumor recurrence was observed in 30 (22%) cases, and 13 (9%) patients died of disease. The median time to death for the uncensored subgroup was 23.7 months (range 1.6–87.1 months), whereas the median follow-up of censored patients was 30.7 months (range 0–180.6 months). The median time to tumor recurrence was 12.7 months (range 0–69.3 months). In univariate analysis, FIGO stage (P<0.0001), presence of lymphovascular invasion (P=0.017) and presence of nodal metastasis (P=0.043) were associated with poor overall survival. D2-40 immunoreactivity within the tumors did not show statistically significant association with overall survival (P=0.137, Figure 3a). On the other hand, presence of lymphovascular invasion (P=0.005), low D2-40 immunoreactivity (P=0.014, Figure 3b) and tumor stage (P=0.043) showed significant association with recurrence-free survival.
FIGO stage, tumor grade, lymphatic invasion, lymph node status and D2-40 immunoreactivity were included in the initial stepwise logistic regression model. Backward elimination by Cox regression led to a model with two independent terms predictive of overall survival: FIGO stage (P<0.001) and lymph node metastasis (P=0.048); and one independent term predictive of recurrence-free survival: lymphovascular invasion (P=0.046).
Discussion
In this study, we characterized the immunohistochemical reactivity of a series of cervical squamous cell carcinomas with the D2-40 monoclonal antibody. The monoclonal antibody D2-40 was recently described to react with a novel oncofetal membrane antigen designated M2A, present on fetal gonocytes, intratubular germ-cell neoplasia and seminoma cells.18 The M2A antigen has also been shown to be a developmental marker for human Sertoli cells, present on immature Sertoli cells until puberty, and lost during their transition to a mature adult phenotype.19 Subsequent studies have indicated that D2-40 immunoreactivity may serve as a new selective marker for lymphatic endothelium, and can be useful in identifying the presence of lymphatic invasion in various malignant neoplasms,14 including cervical carcinoma. However, the reactivity of the tumor cells themselves with D2-40 has not yet been widely evaluated.
Benign cervical squamous epithelia showed moderate-to-strong D2-40 reactivity restricted to the basal cell layer. D2-40 immunoreactivity was significantly increased in carcinomas in situ compared to benign epithelia, while invasive squamous cell carcinomas showed a wide spectrum of immunoreactivity. The majority of tumors (59%) showed focal D2-40 staining, while no reactivity and diffuse staining were seen in 29 and 12%, respectively. In cases with focal immunostaining, reactivity was predominantly localized to the periphery of tumor cell nests, mimicking the basal cell layer localization pattern seen in benign epithelia. In addition, stromal cells associated with invasive (or in situ) tumors showed significantly higher D2-40 immunoreactivity compared to cervical stroma not associated with tumor. As reported previously,14 the D2-40 antibody specifically and strongly labeled lymphatic endothelial cells, highlighting the presence of lymphatic invasion in the tumors. Endothelial cells of blood vessels were negative for D2-40 immunoreactivity. Tumor emboli within lymphatic spaces, as well as metastatic tumor cells in lymph nodes, showed no D2-40 staining in the vast majority of tumors, even in cases when the main tumor mass was D2-40 positive.
D2-40 immunoreactivity in the tumors did not show any association with various clinicopathologic tumor features including age, tumor size, grade, depth of invasion and FIGO stage. On the other hand, low levels of D2-40 immunoreactivity in the tumor cells were significantly associated with the presence of lymphatic invasion and lymph node metastasis. In univariate analysis, low D2-40 reactivity was also associated with a significantly shorter recurrence-free survival, but not with disease-related overall survival. In multivariate analysis, D2-40 reactivity was not a significant predictor of either recurrence-free or disease-related survival.
Prior studies have shown that the M2A antigen (specifically detected by D2-40) is expressed in several human cancer cell lines, including ovarian epithelial carcinomas and bladder transitional cell carcinomas, but is absent in many other cell lines derived from a variety of human tumors.13 It is unclear from these studies whether the expression of M2A in some human cancer cell lines is indicative of a lineage-specific marker shared by the cell line and the tumor from which it was derived, or alternatively, the presence of the antigen represents an aberrant expression of the protein by the transformed cell line in vitro.13 Our results suggest that the expression of this antigen may be a specific property of some human cancers rather than an ‘artifact’ induced by in vitro cultivation of tumor cells.
The M2A antigen, recognized by the D2-40 antibody, is an O-linked sialoglycoprotein exposed on the cell surface.13 Although its function is yet unclear, it contains a simple mucin-type carbohydrate structure, common in mucin-type glycoproteins that are expressed widely on human normal cells and tumors.20, 21, 22 Mucins are large, highly glycosylated proteins recognized by their tandem repeat domains, which are rich in serine and threonine sites for O-glycosylation.23 In recent years, membrane-associated mucins have received increasing attention, first as components of carcinoma cell surfaces,24, 25 and later for their roles in the protection of epithelia and other cells.25, 26 In addition to their role in providing barriers that can limit direct access of other cells26 or large molecules27, 28 to the associated cell surface, recent studies have also implicated them in cellular signaling. By activating growth factor receptors, such as ErbB2,29 or serving as docking sites for intracellular proteins,30 they have been shown to alter cellular behavior, repress apoptosis, enhance proliferative signaling or lead to diminished cell–cell adhesion.26, 29, 31, 32, 33 Given the significant correlation of low D2-40 immunoreactivity with lymphatic invasion, it is intriguing to hypothesize that the M2A antigen may also have functions affecting cellular behavior, such as cell–cell adhesion or invasiveness. Further studies are necessary to address these possibilities.
The lymphatic system is the primary pathway of metastasis for most human cancers, and the extent of lymph node involvement is a key prognostic factor for the patient's outcome. While the importance of the lymphatic system as a pathway for metastasis has been well recognized, there is very little information available about the mechanisms by which tumor cells interact with the lymphatics. Our results suggest that the M2A antigen may play a role in the interaction of tumor cells with the lymphatics, and further study of its potential role may shed light on the mechanisms of the lymphatic spread of tumors.
The presence of lymphovascular invasion is a highly significant risk factor for tumor recurrence in cervical squamous cell carcinoma.6, 7, 8, 9 While some patients with disease found to be limited to the cervix and upper vagina by physical examination are candidates for radical surgical resection of the main tumor mass, other patients undergoing biopsy for confirmation of diagnosis have clearly unresectable disease. These patients are treated primarily by radiation and chemotherapy, and do not undergo surgical removal of the main tumor. However, initial biopsy material may not provide reliable histopathologic features to predict the presence of lymphatic space involvement in the body of the tumor. We found that tumors showing low D2-40 immunoreactivity have 76 and 37% possibility of lymphatic space involvement and nodal metastasis, respectively. In contrast, the corresponding possibilities for tumors with high D2-40 immunoreactivity were 30 and 17%, respectively. Thus, our results suggest that D2-40 immunostaining may serve as a useful marker to predict the presence of lymphatic invasion and increased risk of recurrence based on initial cervical biopsy material alone. Further studies are needed to address this possibility.
In summary, we have characterized the immunoreactivity of cervical squamous cell carcinomas with the D2-40 antibody, which reacts specifically with the M2A antigen. We found a significant correlation between low D2-40 immunostaining in the tumor cells and the presence of lymphatic invasion and lymph node metastasis. Our studies suggest that the M2A antigen may play a role in the interaction of tumor cells with the lymphatics. Examination of D2-40 immunoreactivity in initial cervical biopsy material may be a useful marker to predict lymphovascular invasion and increased risk of recurrence.
References
Folkman J . Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15–18.
Abulafia O, Triest WE, Sherer DM . Angiogenesis in malignancies of the female genital tract. Gynecol Oncol 1999;72:220–231.
Bremer GL, Tiebosch AT, van der Putten HW, et al. Tumor angiogenesis: an independent prognostic parameter in cervical cancer. Am J Obstet Gynecol 1996;174:126–131.
Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993;362:841–844.
Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8.
Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352–357.
Creasman WT, Kohler MF . Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol Oncol 2004;92:525–529.
Waggoner SE . Cervical cancer. Lancet 2003;361:2217–2225.
Tsai CS, Lai CH, Wang CC, et al. The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and postoperative radiotherapy. Gynecol Oncol 1999;75:328–333.
Clarijs R, Ruiter DJ, de Waal RM . Lymphangiogenesis in malignant tumours: does it occur? J Pathol 2001;193:143–146.
Karpanen T, Alitalo K . Lymphatic vessels as targets of tumor therapy? J Exp Med 2001;194:F37–F42.
Sleeman JP, Krishnan J, Kirkin V, et al. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech 2001;55:61–69.
Marks A, Sutherland DR, Bailey D, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer 1999;80:569–578.
Kahn HJ, Marks A . A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest 2002;82:1255–1257.
FIGO stages (announcements)—1988 revision. Gynecol Oncol 1989;35:125–127.
Kurman RJ, Norris HJ, Wilkinson EJ . Tumors of the Cervix, Vagina, and Vulva. Armed Forces Institute of Pathology: Washington, DC, 1992.
Acs G, Lawton TJ, Rebbeck TR, et al. Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 2001;115:85–98.
Bailey D, Baumal R, Law J, et al. Production of a monoclonal antibody specific for seminomas and dysgerminomas. Proc Natl Acad Sci USA 1986;83:5291–5295.
Baumal R, Bailey D, Giwercman A, et al. A novel maturation marker for human Sertoli cells. Int J Androl 1989;12:354–359.
Sutherland DR, Rudd CE, Greaves MF . Isolation and characterization of a human T lymphocyte-associated glycoprotein (gp40). J Immunol 1984;133:327–333.
Carraway KL, Hull SR . O-glycosylation pathway for mucin-type glycoproteins. Bioessays 1989;10:117–121.
Lesuffleur T, Zweibaum A, Real FX . Mucins in normal and neoplastic human gastrointestinal tissues. Crit Rev Oncol Hematol 1994;17:153–180.
Strous GJ, Dekker J . Mucin-type glycoproteins. Crit Rev Biochem Mol Biol 1992;27:57–92.
McNeer RR, Huang D, Fregien NL, et al. Sialomucin complex in the rat respiratory tract: a model for its role in epithelial protection. Biochem J 1998;330:737–744.
Gendler SJ, Spicer AP . Epithelial mucin genes. Annu Rev Physiol 1995;57:607–634.
Carraway KL, Perez A, Idris N, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol 2002;71:149–185.
Komatsu M, Yee L, Carraway KL . Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res 1999;59:2229–2236.
Price-Schiavi SA, Jepson S, Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer 2002;99:783–791.
Klapper LN, Kirschbaum MH, Sela M, et al. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res 2000;77:25–79.
Carraway KL, Ramsauer VP, Haq B, et al. Cell signaling through membrane mucins. Bioessays 2003;25:66–71.
Komatsu M, Jepson S, Arango ME, et al. Muc4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene 2001;20:461–470.
Yamamoto M, Bharti A, Li Y, et al. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem 1997;272:12492–12494.
Wesseling J, van der Valk SW, Vos HL, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 1995;129:255–265.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 93rd Annual Meeting of the United States and Canadian Academy of Pathology, Vancouver, BC, Canada, March 6–12, 2004.
Rights and permissions
About this article
Cite this article
Dumoff, K., Chu, C., Xu, X. et al. Low D2-40 immunoreactivity correlates with lymphatic invasion and nodal metastasis in early-stage squamous cell carcinoma of the uterine cervix. Mod Pathol 18, 97–104 (2005). https://doi.org/10.1038/modpathol.3800269
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800269
Keywords
This article is cited by
-
The prognostic values of the expression of Vimentin, TP53, and Podoplanin in patients with cervical cancer
Cancer Cell International (2017)
-
Expression of HNF-1β in cervical carcinomas: an immunohistochemical study of 155 cases
Diagnostic Pathology (2015)
-
Expression of podoplanin in the invasion front of oral squamous cell carcinoma is not prognostic for survival
Virchows Archiv (2015)
-
Podoplanin: a novel regulator of tumor invasion and metastasis
Medical Oncology (2014)
-
Targeting therapy of hepatocellular carcinoma with doxorubicin prodrug PDOX increases anti-metastatic effect and reduces toxicity: a preclinical study
Journal of Translational Medicine (2013)