Abstract
The over-representation of chromosome 12p sequences is crucial for the development of invasive testicular germ cell tumors. Testicular cancer patients may have metastatic tumors of diverse histologic types, including adenocarcinoma, undifferentiated carcinoma, sarcoma, or other malignancies that lack features of germ cell tumors. We sought to investigate the possible germ cell origin of such tumors using interphase fluorescence in situ hybridization. In all, 10 metastatic malignant somatic-type tumors from patients with histories of testicular cancer, as well as one malignant somatic-type tumor from a patient with primary mediastinal germ cell tumor were studied and included: adenocarcinoma (five cases), poorly differentiated carcinoma (one), sarcoma (four), and neuroendocrine carcinoma (one). The tumors were analyzed using fluorescence in situ hybridization using 12p spectrum green and 12 centromeric spectrum orange probes in paraffin sections. The patients ranged in age from 27 to 55 years (mean, 43). Colon and lung cancers from patients without germ cell tumors were used as controls. Adequate signals were observed in all tumors. Gain of chromosome 12p was seen in six tumors. None of the control tumors showed 12p amplification. Fluorescence in situ hybridization for 12p amplification in routinely processed surgical specimens is a useful adjuvant diagnostic tool in confirming the germ cell origin of metastatic tumors having the histologic appearance of somatic-type neoplasms.
Similar content being viewed by others
Main
Germ cell tumor of the testis is the most common solid tumor malignancy in young adult men.1 Testicular germ cell tumors are often comprised of a variety of histological subtypes, with strong evidence supporting a common clonal origin.2 Isochromosome 12p (i12p) and other forms of 12p amplification are seen in the vast majority of testicular germ cell tumors of adults and are present in all histological subtypes.
Malignant germ cell tumors of the testis frequently metastasize to lymph nodes in the retroperitoneum and mediastinum as well as other locations. Most often these lesions resemble the original testicular primary tumor or have a pattern of a germ cell tumor type that was not seen in the primary tumor. Rarely, these metastatic lesions will present as somatic-type malignancies, taking the form of a carcinoma or sarcoma. In such cases, the diagnosis of a germ cell origin of the metastatic lesion is made based on the known history and lack of any other primary malignancy which could account for the metastatic lesion. Careful examination of the metastatic lesion in these tumors may occasionally show a small component resembling germ cell tumor. However, in very rare instances, these somatic-type malignancies of germ cell origin may occur as primary mediastinal lesions, or in the absence of a known testicular primary because of spontaneous regression (‘burned-out’ germ cell tumor). Therefore, there is a need for a test that can serve as an adjuvant diagnostic tool for establishing the germ cell origin in such cases.
In this study, we sought to evaluate two-color interphase fluorescent in situ hybridization using four micron unstained paraffin-embedded tissue slides to detect 12p amplification. We examined specimens from patients with histories of germ cell tumors, who later presented with metastatic disease in the form of somatic-type malignant lesions.
Materials and methods
Specimens
In all, 11 metastatic malignant somatic type tumors were evaluated using fluorescence in situ hybridization (FISH). The patients' ages at the time of diagnosis ranged from 27 to 55 years (mean, 43 years). In one patient, two separate lesions with different histologic characteristics, separated in presentation by 3 years, were examined. Eight of the 10 patients had histories of testicular germ cell tumors. One of the 10 patients had regressed testicular germ cell tumor (scar only) at the time of orchiectomy. One patient had a primary mediastinal germ cell tumor.
Tissue Preparation and FISH
From each specimen, multiple 4 μm unstained sections were made from paraffin-embedded tissue blocks. The paraffin blocks had been stored for periods ranging from a few months to 17 years. A hematoxylin and eosin-stained slide from each specimen was examined for determination of areas for cell counting. The unstained slides were deparaffinized with two 10 min xylene washes. The slides were then hydrated using 100, 85, and 70% ethanol solution washes for 10 min each, followed by 10 min in distilled water, and two 10 min rinses in phosphate buffered solution at pH 7. After hydration, the slides were placed in a methanol–acetic acid solution (3:1 ratio) for 10 min then air dried. The slides were incubated at 85°C for 1 h in 0.1 mM citric acid (pH 6) solution. The specimen was then digested with 0.75 ml of a pepsin (Sigma, St Louis, MO, USA) solution (5 mg/ml in 0.9% NaCl at pH 1.5) in a humidified box for 30 min at 37°C. The slides were then briefly rinsed with distilled water, dehydrated in a series of ethanol solutions and allowed to air dry.3, 4
Probes for 12p spectrum green and 12 centromeric spectrum orange (Vysis, Downers Grove, IL, USA) were diluted by a ratio of 1:50 with tDenHyb2 buffer (Insitus, Alburquerque, NM, USA). A volume of 10 μl of the diluted probed was applied to the slides in the area of interest. The slide was covered with a glass coverslip and sealed with rubber cement. The slides were placed in an opaque plastic box wrapped with aluminum foil. Denaturation was achieved by incubating the slides at 80°C for 10 min, after which hybridization was performed at 37°C for 16–18 h. The coverslips were removed and 10 μl of DAPI (Insitus, Albuquerque, NM, USA) was applied to each slide. A new coverslip was placed and the slides were examined with an Olympus IX-50 microscope (Olympus, Tokyo, Japan). Metastatic embryonal carcinoma from a patient with a history of testicular germ cell tumor was used as a positive control. For negative controls, primary lung and colon adenocarcinomas from patients without histories of germ cell tumors were used.
Criteria for signal detection were described previously.3, 4 In all, 40 cells were counted for each case. Patterned after the criteria for HER2/neu amplification, the green signal (12p) and orange signal (12 centromeric) were counted for each tumor cell nucleus, and the average number of each signal was recorded.5 We considered a 12p/12 centromeric ratio of 1.5 or greater as evidence of 12p amplification.
Results
We observed 12p amplification in six of 11 cases (Table 1), including three adenocarcinomas (Figure 1), two sarcomas, and one neuroendocrine carcinoma. Those six patients' ages ranged from 27 to 55 years. The time from initial diagnosis of germ cell tumor to the presentation of the metastatic somatic-type tumor in the patients with 12p amplification ranged from 5 to 16 years. One patient had metastases separated by 3 years and both tumors showed 12p amplification. The patient with a primary mediastinal germ cell tumor, in the absence of known testicular primary, did not have 12p amplification. Of the tumors without 12p amplification, two were adenocarcinomas, one was poorly differentiated carcinoma, and two were sarcoma. The ages of these patients ranged from 39 to 46 years. The control specimens of primary lung and colon carcinoma did not show 12p amplification.
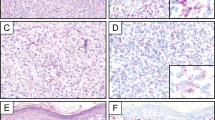
Histologic features of somatic-type malignancy (adenocarcinoma) in a patient with a history of testicular germ cell tumor. (a) Some of the cells in the gland at the bottom show intracytoplasmic mucin (A). Fluorescence in situ hybridization (b) showed two signals (orange) for the chromosome 12 centromeric probe (arrows) and numerous signals for 12p (green).
Discussion
Rarely, tumors of germ cell origin present with histologic features of somatic type malignancies. Often, these occur in patients with a known history of primary testicular germ cell tumor. However, it is possible for these tumors to occur in patients without a known history, or in patients with a history of both testicular germ cell tumor and a secondary known primary tumor such as carcinoma or sarcoma. In such cases, confirmatory tests would be useful for classifying the metastatic tumor as of germ cell origin. We studied metastatic somatic-type malignancies from patients with histories of testicular germ cell tumors. Of these, six of 11 showed 12p amplification.
Abnormalities of 12p have been shown to occur early in the evolution of germ cell tumor, with some evidence that 12p amplification is required for the development of an invasive tumor.6, 7, 8, 9, 10 There are many studies which have evaluated 12p abnormalities in testicular germ cell tumors. These studies include classic cytogenetic analyses, FISH on metaphase cells of fresh tissue, FISH on interphase cells from paraffin-embedded tissue, and comparative genomic hybridization. Standard cytogenetics detects isochromosome 12p i(12p) in only 50–70% of testicular germ cell tumors.11 The i(12p)-negative cases, however, showed other 12p abnormalities.12 Classic cytogenetics has long been the standard method used in studying testicular germ cell tumors. However, recent studies have shown FISH to be more sensitive in identifying 12p abnormalities than conventional cytogenetics. Of 15 testicular germ cell tumors, 14 harbored 12p abnormalities using FISH.7 Another study by Smolarek et al,13 using FISH to evaluate 11 primary germ cell tumors and 16 metastatic germ cell tumors involving lymph nodes, found over-representation of 12p in all 27 tumors. Other studies, including comparative genomic hybridization, have also characterized the overexpression of 12p in testicular germ cell tumors. Mostert et al14 examined 15 primary testicular germ cell tumors and two metastatic germ cell tumors. They found that gain of 12p in the majority of the tumors was observed to involve the complete short arm of chromosome 12. However, in some tumors the amplification was specific to 12p11.1–p12.1.14
Interphase FISH studies have recently been shown to be a valuable technique for the identification of 12p abnormalities in germ cell tumors. Blough et al15 performed a study comparing interphase FISH on paraffin-embedded tissue to metaphase FISH. They studied 14 testicular tumors and found that there were nearly identical results in seven, close agreement in five, and some disparity in two.15 They concluded that interphase FISH studies on paraffin-embedded tissue can reliably detect chromosome 12 abnormalities in testicular germ cell tumors.15 Motzer et al16 also found that identification of 12p abnormalities is useful in establishing germ cell origin in tumors of uncertain histogenesis. They studied eight patients without known primary germ cell tumors who presented with poorly differentiated carcinoma. Four of eight had chromosome abnormalities involving 12p, consistent with a germ cell origin. Three of four subsequently responded to cisplatin-based chemotherapy,16 further supporting the germ cell origin of these tumors. Additionally, comparative genomic hybridization has been used to evaluate tumors of uncertain histogenesis. In one study, tumors from seven patients who were later shown to have primary germ cell tumors were evaluated with comparative genomic hybridization.17 All had gain of 12p, one of which showed a 12p gain restricted to the 12p11.2–p12 region.17
In the rare case of patients with somatic-type metastatic lesions with unknown primary site, or a question of metastatic germ cell tumor vs carcinoma or sarcoma, the identification of germ cell origin would be useful. Our study provides a technique that would allow the use of interphase FISH on readily available paraffin-embedded tissue as an adjuvant diagnostic tool. This technique provides a simple supplemental study in difficult cases. The finding of 12p amplification would strongly support the diagnosis of germ cell origin in cases where this diagnosis is suspected. However, this is a pilot study and there are several limitations. We selected a slide thickness that would prevent significant nuclear overlap (4 μm) while still allowing visualization of much of the nucleus. Inevitably, by using standard thickness sections, not all of the nuclei will contain all of the chromosomes because part of the nucleus will not be present on the slide. This may result in several cells having a decrease in signal count. Nonetheless, interphase FISH has been recently standardized for routine application in surgical pathology, such as Her2/neu test for breast cancer. We found that most tumors had enough areas without overlapping to allow for counting 40 tumor cells. The paraffin blocks used ranged from several months to 17 years in age. Adequate signal was observed even in the oldest cases studied.
Interphase FISH for 12p amplification can provide useful adjuvant diagnostic information by confirming the germ cell origin of somatic-type tumors of uncertain histogenesis.
References
Jemal A, Tiwari RC, Murry T, et al. Cancer statistics, 2004. CA Cancer J Clin 2004;54:8–29.
Kernek KM, Ulbright TM, Zhang S, et al. Identical allelic losses in mature teratoma and other histologic components of malignant mixed germ cell tumors of the testis. Am J Pathol 2003;163:2477–2484.
Brunelli M, Eble JN, Zhang S, et al. Metanephric adenoma lacks the gains of chromosomes 7 and 17 and loss of Y which are typical of papillary renal cell carcinoma and papillary adenoma. Mod Pathol 2003; 16:1060–1063.
Brunelli M, Eble JN, Martignoni G, et al. Gain of chromosomes 7, 17, 12, 16, and 20, and loss of Y occur early in the evolution of papillary renal cell neoplasia: a fluorescent in situ hybridization study. Mod Pathol 2003;16:1053–1059.
Latta EK, Tjan S, Parkes RK, et al. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol 2002;15:1318–1325.
Looijenga LHJ, Oosterhuis JW . Pathogenesis of testicular germ cell tumours. Rev Reprod 1999;4:90–100.
Pienkowska-Grela B, Grygalewicz B, Bregula U . Overrepresentation of the short arm of chromosome 12 in seminoma and nonseminoma groups of testicular germ cell tumors. Cancer Genet Cytogenet 2002;134:102–108.
Samaniego F, Rodriguez E, Houldsworth J, et al. Cytogenetic and molecular analysis of human male germ cell tumors: chromosome 12 abnormalities and gene amplification. Genes Chromosomes Cancer 1990;1:289–300.
Rodriguez E, Houldsworth J, Reuter VE, et al. Molecular cytogenetic analysis of i(12p)-negative human male germ cell tumors. Genes Chromosomes Cancer 1993;8:230–236.
Suijkerbuijk RF, Sinke RJ, Weghuis DE, et al. Amplification of chromosome subregion 12p11.2–p12.1 in a metastasis of an i(12p)-negative seminoma: relationship to tumor progression? Cancer Genet Cytogenet 1994;78:145–152.
Chaganti RSK, Houldsworth J . Genetics and biology of adult human male germ cell tumors. Cancer Res 2000;60:1475–1482.
Geurts van Kessel A, Suijkerbuijk RF, Sinke RJ, et al. Molecular cytogenetics of human germ cell tumours: i(12p) and related chromosomal anomalies. Eur Urol 1993;23:23–29.
Smolarek TA, Blough RI, Foster RS, et al. Identification of multiple chromosome 12 abnormalities in human testicular germ cell tumors by two-color fluorescence in situ hybridization (FISH). Genes Chromosomes Cancer 1995;14:252–258.
Mostert MMC, van de Pol M, Olde Weghuis D, et al. Comparative genomic hybridization of germ cell tumors of the adult testis: confirmation of karyotypic findings and identification of a 12p-amplicon. Cancer Genet Cytogenet 1996;89:146–152.
Blough RI, Smolarek TA, Ulbright TM, et al. Bicolor fluorescence in situ hybridization on nuclei from formalin-fixed, paraffin-embedded testicular germ cell tumors: comparison with standard metaphase analysis. Cancer Genet Cytogenet 1997;94:79–84.
Motzer RJ, Rodriquez E, Reuter VE, et al. Genetic analysis as an aid in diagnosis for patients with midline carcinomas of uncertain histologies. J Natl Cancer Inst 1991;83:341–346.
Summersgill B, Goker H, Osin P, et al. Establishing germ cell origin of undifferentiated tumors by identifying gain of 12p material using comparative genomic hybridization analysis of paraffin-embedded samples. Diag Mol Pathol 1998;7:260–266.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented, in part, at the 92nd annual meeting of the United States and Canadian Academy of Pathology, Washington, DC, March 22–28, 2003.
Rights and permissions
About this article
Cite this article
Kernek, K., Brunelli, M., Ulbright, T. et al. Fluorescence in situ hybridization analysis of chromosome 12p in paraffin-embedded tissue is useful for establishing germ cell origin of metastatic tumors. Mod Pathol 17, 1309–1313 (2004). https://doi.org/10.1038/modpathol.3800195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800195
Keywords
This article is cited by
-
Hodentumoren aus klinischer Sicht
Die Pathologie (2022)
-
Molecular correlates of male germ cell tumors with overgrowth of components resembling somatic malignancies
Modern Pathology (2022)
-
Was der Onkologe vom Pathologen über Hodentumoren wissen muss
Der Pathologe (2020)
-
Histology, 12p status, and IMP3 expression separate subtypes in testicular teratomas
Virchows Archiv (2020)
-
Retroperitoneal teratoma with somatic malignant transformation: A papillary renal cell carcinoma in a testicular germ cell tumour metastasis following platinum-based chemotherapy
BMC Urology (2013)