Abstract
Primary small cell carcinomas of the uterine cervix are uncommon but highly aggressive malignancies. The recent observations that c-kit proto-oncogene, a tyrosine kinase, is overexpressed in small cell lung cancers and that advanced c-kit-expressing gastrointestinal stromal tumors were successfully treated with a selective tyrosine kinase inhibitor STI-571 (Gleevec, imatinib mesylate) prompted us to investigate c-kit protein expression in cervical small cell carcinomas. Using a polyclonal antibody against c-kit protein (CD117), our immunohistochemical studies demonstrated a cytoplasmic staining in six of 22 cases (27%) of cervical small cell carcinoma. However, in five of these c-kit-positive cases, the immunoreactivity was focal and/or weak. Only one case (5%) exhibited a strong and diffuse staining pattern comparable to that seen in gastrointestinal stromal tumors. This was in contrast to small cell lung cancers where a positive staining for c-kit was observed in nine of 14 cases (64%) included in the study for comparison. Among them, five (36%) exhibited a strong and diffuse staining pattern. These results indicate that overexpression of c-kit protein is an infrequent event in small cell carcinomas of the uterine cervix. In comparison with small cell lung cancers, the findings presented in this report may reflect the difference in etiopathogenetic mechanisms underlying these two types of small cell carcinomas.
Similar content being viewed by others
Main
The c-kit proto-oncogene encodes a transmembrane tyrosine kinase receptor (CD117) that is structurally related to several other tyrosine kinase receptors such as the platelet-derived growth factor receptors.1 It is universally expressed in gastrointestinal stromal tumor, the most common mesenchymal neoplasm of the gastrointestinal tract, with only rare exceptions.2, 3 In fact, immunohistochemical detection of c-kit protein expression has become one of the most important criteria in the diagnosis of gastrointestinal stromal tumor. In the majority of gastrointestinal stromal tumors, the expression is associated with mutations of the c-kit gene, which lead to constitutive activation of the receptor kinase activity in a ligand-independent manner, thus providing uncontrolled growth-promoting and antiapoptotic signals for tumor cells.4 These observations have revolutionized the clinical management of gastrointestinal stromal tumors. Multicenter trials have demonstrated a partial response in the majority of patients with advanced gastrointestinal stromal tumors treated with STI-571 (Gleevec, imatinib mesylate), a small compound that selectively inhibits the activity of a limited number of receptor tyrosine kinases including c-kit.5, 6, 7, 8
In addition to gastrointestinal stromal tumor, overexpression of c-kit protein has been reported in several human malignancies,8, 9, 10, 11, 12 notably small cell lung cancer. A number of studies have shown that c-kit protein is overexpressed in 28–91% of human small cell lung cancer specimens as determined by immunohistochemistry.10, 11, 12, 13, 14, 15, 16, 17 In contrast to gastrointestinal stromal tumor, the expression of c-kit protein in small cell lung cancer does not appear to be associated with c-kit gene mutations.17, 18 Instead, coexpression of c-kit and its ligand stem cell factor (also known as steel factor) has been demonstrated in approximately 70% of small cell lung cancer cell lines, suggesting an autocrine mechanism, which may be important for small cell lung cancer pathogenesis.19, 20, 21, 22, 23 Interestingly, recent studies have shown that selective tyrosine kinase inhibitor STI-571 also inhibits the growth of small cell lung cancer cells in vitro through a mechanism that inactivates the kinase activity of c-kit.24, 25 These experimental findings may have potential therapeutic implications despite the recent disappointing results of a phase II clinical trial.26 It should be pointed out, however, that this small trial enrolled only 19 patients, among which five were actually not of small cell type. In the remaining 14 cases of small cell lung cancer, only four (29%) expressed c-kit by immunohistochemistry.26
The current study was specifically designed to investigate c-kit protein expression in small cell carcinomas of the uterine cervix, a rare but highly aggressive neoplasm.27, 28, 29, 30, 31 Our results demonstrate that unlike small cell lung cancers, cervical small cell carcinomas did not frequently overexpress this potentially important therapeutic target protein.
Materials and methods
Case Selection
A total of 22 primary small cell carcinomas of the uterine cervix were retrieved from the 1989–2002 surgical pathology archives at Washington University Medical Center. These included 12 hysterectomies and 10 biopsies. Formalin-fixed, paraffin-embedded tissue blocks were available for each case. Hematoxylin and eosin (H&E)-stained slides were re-examined to confirm the original diagnosis. For inclusion in the study, a tumor was required to exhibit the typical morphology of a small cell carcinoma identical to that seen in its pulmonary counterpart.32 In addition, more than 50% of the tumor cells were required to express one or more of the neuroendocrine markers including chromogranin, synaptophysin or neuron-specific enolase by immunohistochemistry. Clinical data were reviewed to rule out the remote possibility of metastasis from a lung or other primary.
In addition, 14 small cell lung cancers and 15 gastrointestinal stromal tumors were included in the study for comparison. Formalin-fixed, paraffin-embedded tissue blocks and H&E-stained slides were available for each case.
Immunohistochemical Analysis
Immunohistochemical staining was performed on 4-μm tissue sections employing the LSAB Plus system (Dako Corp., Carpinteria, CA, USA) and the ABC kit (Vector Laboratories, Burlingame, CA, USA) following the manufacturers' instructions with slight modifications. Specifically, deparaffinized tissue sections were first treated with 3% H2O2 for 15 min to inhibit endogenous peroxidase activity and then subjected to antigen retrieval by heating in 1 mmol/l EDTA (pH 8.0) for 4 min. After incubation with blocking serum for 20 min, sections were incubated with a rabbit polyclonal antibody against c-kit (CD117; catalog no. A4502) obtained from Dako Corp. for 1 h at room temperature with an antibody dilution of 1:200. After further incubation with biotinylated link antibodies and peroxidase-labeled streptavidin, the staining was developed by reaction with DAB substrate-chromogen solution followed by counterstaining with hematoxylin. In each experiment, a negative control was included in which the primary antibody was replaced by preimmune rabbit IgG.
Cytoplasmic (with or without membranous) staining was considered positive for c-kit protein expression. Each tumor was scored as completely negative (<5% of the tumor cells positive), 1+ (5–25%), 2+ (26–50%) or 3+ (>50%), as well as weak or strong according to the staining intensity where ‘strong’ was defined as the intensity comparable to that seen in gastrointestinal stromal tumors.
Statistical Analysis
Statistical analysis was carried out employing the SAS system. A P value of <0.05, as determined by the χ2 test or two-tailed Fisher's exact test when applicable, was considered statistically significant.
Results
Clinicopathologic Features of Cervical Small Cell Carcinomas
The patients with primary small cell carcinomas of the uterine cervix included in our study ranged in age from 19 to 79 years (mean 40. 5 years, median 38.5 years). They were in general younger than those with small cell lung cancer, whose ages ranged from 50 to 84 years, with a mean age of 65.7 years and a median age of 64 years.
At the time of diagnosis, 16 cervical tumors (73%) were FIGO stage IB, 2 stage IA, 3 stage IIIA and 1 stage IIIB. One case had regional nodal metastasis and one had documented distal metastasis. The tumor size in 12 hysterectomy specimens ranged from 1 to 12 cm (mean 3.7 cm, median 2.8 cm).
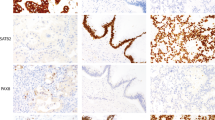
Histologically, small cell carcinomas of the uterine cervix exhibited morphologic features identical to those seen in small cell lung cancers. Typically, the tumors were composed of sheets of small round or ovoid, or intermediate-sized short-spindle cells with hyperchromatic nuclei, no or inconspicuous nucleoli, scanty cytoplasm, and a very high nuclear-to-cytoplasmic ratio (Figure 1). Nested and trabecular growth patterns with peripheral palisading and rosette formation were appreciated in some of the cases. The tumors also exhibited brisk mitotic activity and individual cell necrosis (apoptosis). Large areas of coagulative necrosis were present in a few cases. In biopsy specimens, crush artifact in tumor cells was evident.
All the cervical tumors included in the study were immunohistochemically positive for at least one neuroendocrine marker, with the positivity frequency being 72, 72 and 68% for chromogranin, synaptophysin and neuron-specific enolase, respectively. The majority of the tumors (68%) were positive for two of the markers, and the remaining cases were positive for one or three markers (9 and 23%, respectively). In the 19 cases where immunostaining for pancytokeratin was performed, seven exhibited a characteristic paranuclear dot-like staining pattern. One tumor was also subjected to electron microscopic examination, which demonstrated the presence of cytoplasmic neurosecretory granules.
Analysis of c-kit Protein Expression by Immunohistochemistry
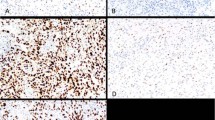
Table 1 summarizes the results of immunohistochemical studies on c-kit protein expression in cervical small cell carcinomas and compares with the findings in small cell lung cancers and gastrointestinal stromal tumors. As positive controls, all 15 gastrointestinal stromal tumors showed strong and diffuse cytoplasmic c-kit immunostaining. However, there were only six cervical small cell carcinomas (27%) that exhibited variable immunoreactivity to the anti-c-kit antibody. Since c-kit immunoreactivity seen in gastrointestinal stromal tumors was always strong and diffuse (Figure 2a), we chose to only consider the cases with a 3+/strong staining pattern as truly positive (Figure 2b), which was observed in only one of 22 cervical tumors (5%). The cases that were either completely negative (Figure 3a) or exhibited focal (1+ or 2+) or weak staining (Figure 3b) were all considered as negative. No detectable c-kit immunostaining was appreciated in non-neoplastic ectocervical squamous or endocervical glandular epithelium present in some of the sections (Figure 4).
The immunohistochemical findings from cervical small cell carcinomas were significantly different than those from small cell lung cancers. As shown in Table 2, nine of 14 small cell lung cancers (64%) showed some degree of c-kit immunoreactivity and five (36%) exhibited a staining pattern similar to that seen in gastrointestinal stromal tumors characterized by strong and diffuse cytoplasmic positivity.
Discussion
The successful treatment of advanced gastrointestinal stromal tumors with tyrosine kinase inhibitor STI-571 has raised the hope that other malignancies could also be similarly treated. One of the potential candidates would be small cell lung cancer since a large subset of the tumors have been demonstrated to overexpress c-kit protein10, 11, 12, 13, 14, 15, 16, 17 and since in vitro studies have shown promising results.24, 25 Although the recent phase II clinical trial showed no objective response in a small number of small cell lung cancer patients treated with STI-571, the final answer has to wait for new trials that will include more patients with c-kit-expressing tumors.26
Primary small cell carcinoma of the uterine cervix is an uncommon malignancy, accounting for 0.5–2% of all cervical cancers.29, 30 Similar to small cell lung cancer, cervical small cell carcinoma is difficult to manage and usually follows an aggressive clinical course, with death within a few years after diagnosis.24, 25, 26, 27, 28, 29, 30, 31 Unlike small cell lung cancer, however, the expression of c-kit protein in cervical small cell carcinomas has not been well investigated. In fact, there has been only one study to date that has included two cases of cervical small cell carcinoma. By immunohistochemistry, both cases showed negative c-kit expression.11
In this report, we demonstrate that overexpression of c-kit protein is an infrequent event in cervical small cell carcinomas. Among 22 cases we studied, only one case exhibited the characteristic staining pattern similar to that seen in gastrointestinal stromal tumors, although variable c-kit immunoreactivity was noted in other five cases. These observations thus suggest that the majority of the patients with cervical small cell carcinomas would be unlikely to benefit from STI-571 therapy, even if the future clinical trials for small cell lung cancers were successful.
An important concern on detecting c-kit protein expression by immunohistochemistry is nonspecific staining. This critical issue has been the focus of a number of recent publications, particularly regarding c-kit expression in desmoid fibromatosis,33, 34, 35, 36, 37, 38 and should also be relevant to the studies on small cell lung cancers. In this regard, we demonstrate that a significant fraction of c-kit-positive small cell lung cancers exhibited only weak and/or focal staining. These observations are similar to what has been documented in the literature.10, 12, 13, 17. For example, Naeem et al13 reported that 16 of 30 small cell lung cancers (53%) were immunohistochemically positive for c-kit expression, but in only four cases (13%) was the staining intensity equal to that in gastrointestinal stromal tumors. In the study by Burger et al,17 14 out of 22 small cell lung cancers (64%) were demonstrated to be c-kit-positive, among which nine (41%) showed moderate to strong staining whereas the remaining five were weakly positive. It is our opinion that the cases with weak and/or focal staining should be better considered as negative or nonspecific, and that the designation of positive c-kit expression should be reserved only for those with staining extent and intensity comparable to gastrointestinal stromal tumors. This may explain, in part, why STI-571 is not as effective for small cell lung cancers as for gastrointestinal stromal tumors.26 although lack of mutations at exon 11 of the c-kit gene in small cell lung cancers may be another explanation.17
An interesting finding in this study is that the frequency of c-kit protein overexpression in cervical small cell carcinomas is significantly lower than that in their pulmonary counterparts. Although the exact underlying mechanisms remain to be investigated, we hypothesize that this difference may be related to different etiopathogenetic mechanisms involved in these two types of malignancies in spite of their identical morphology. It has been well established that small cell carcinomas of the uterine cervix, usually occurring in younger women around 40 years of age,27, 28, 29, 30, 31, 39, 40, 41 is etiologically associated with high-risk types of human papillomavirus.39, 40, 41, 42, 43, 44, 45 In contrast, small cell lung cancer is associated with cigarette smoking and usually occurs in older individuals.46, 47, 48 In support of this hypothesis, the patients with small cell lung cancers included in this study are 25 years older, on an average, than those with cervical small cell carcinomas. In addition, all cervical small cell carcinomas in this study are found to harbor high-risk human papillomavirus DNA, with type 18 being most prevalent (HL Wang and DW Lu, unpublished observations). Furthermore, the molecular mechanisms by which the tumor suppressor genes p53 and Rb are deregulated also appear to be different in these two types of small cell carcinomas. In small cell lung cancers, deregulation occurs at the genetic level via gene mutations,49, 50, 51, 52 whereas in cervical tumors, the tumor suppressor functions of these two genes are inactivated most likely at the protein level through interaction with the E6 and E7 oncoproteins of the papillomavirus.53, 54, 55, 56, 57
Our observations that c-kit protein is overexpressed in only a small subset of small cell carcinomas (in both the cervix and the lung) suggest that this event is not an initiating step in tumor development as proposed for gastrointestinal stromal tumor.4, 58 Rather, it may represent one of the molecular abnormalities accumulated during tumor progression. This may help explain why many small cell carcinomas do not show c-kit protein expression while others exhibit only focal, instead of diffuse, immunoreactivity.
References
Gibson PC, Cooper K . CD117 (KIT): a diverse protein with selective applications in surgical pathology. Adv Anat Pathol 2002;9:65–69.
Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459–465.
Miettinen M, Majidi M, Lasota J . Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 2002;38(Suppl 5):S39–S51.
Heinrich MC, Rubin BP, Longley BJ, et al. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol 2002;33:484–495.
Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 2002;33:466–477.
Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480.
Radford IR . Imatinib. Novartis. Curr Opin Investig Drugs 2002;3:492–499.
Heinrich MC, Blanke CD, Druker BJ, et al. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol 2002;20:1692–1703.
Natali PG, Nicotra MR, Sures I, et al. Expression of c-kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res 1992;52:6139–6143.
Matsuda R, Takahashi T, Nakamura S, et al. Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol 1993;142:339–346.
Tsuura Y, Hiraki H, Watanabe K, et al. Preferential localization of c-kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminoma/dysgerminoma in human: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Virchows Arch 1994;424:135–141.
Arber DA, Tamayo R, Weiss LM . Paraffin section detection of the c-kit gene product (CD117) in human tissues: value in the diagnosis of mast cell disorders. Hum Pathol 1998;29:498–504.
Naeem M, Dahiya M, Clark JI, et al. Analysis of c-kit protein expression in small-cell lung carcinoma and its implication for prognosis. Hum Pathol 2002;33:1182–1187.
Micke P, Basrai M, Faldum A, et al. Characterization of c-kit expression in small cell lung cancer: prognostic and therapeutic implications. Clin Cancer Res 2003;9:188–194.
Potti A, Moazzam N, Ramar K, et al. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol 2003;14:894–897.
Lonardo F, Pass HI, Lucas DR . Immunohistochemistry frequently detects c-Kit expression in pulmonary small cell carcinoma and may help select clinical subsets for a novel form of chemotherapy. Appl Immunohistochem Mol Morphol 2003;11:51–55.
Burger H, den Bakker MA, Stoter G, et al. Lack of c-kit exon 11 activating mutations in c-KIT/CD117-positive SCLC tumour specimens. Eur J Cancer 2003;39:793–799.
Sekido Y, Takahashi T, Ueda R, et al. Recombinant human stem cell factor mediates chemotaxis of small-cell lung cancer cell lines aberrantly expressing the c-kit protooncogene. Cancer Res 1993;53:1709–1714.
Hibi K, Takahashi T, Sekido Y, et al. Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene 1991;6:2291–2296.
Sekido Y, Obata Y, Ueda R, et al. Preferential expression of c-kit protooncogene transcripts in small cell lung cancer. Cancer Res 1991;51:2416–2419.
Rygaard K, Nakamura T, Spang-Thomsen M . Expression of the proto-oncogenes c-met and c-kit and their ligands, hepatocyte growth factor/scatter factor and stem cell factor, in SCLC cell lines and xenografts. Br J Cancer 1993;67:37–46.
Plummer III H, Catlett J, Leftwich J, et al. c-myc expression correlates with suppression of c-kit protooncogene expression in small cell lung cancer cell lines. Cancer Res 1993;53:4337–4342.
Krystal GW, Hines SJ, Organ CP . Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res 1996;56:370–376.
Krystal GW, Honsawek S, Litz J, et al. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res 2000;6:3319–3326.
Wang WL, Healy ME, Sattler M, et al. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene 2000;19:3521–3528.
Soria JC, Johnson BE, Chevalier TL . Imatinib in small cell lung cancer. Lung Cancer 2003;41(Suppl 1):S49–S53.
Gersell DJ, Mazoujian G, Mutch DG, et al. Small-cell undifferentiated carcinoma of the cervix: a clinicopathologic, ultrastructural, and immunocytochemical study of 15 cases. Am J Surg Pathol 1988;12:684–698.
Perrin L, Ward B . Small cell carcinoma of the cervix. Int J Gynecol Cancer 1995;5:200–203.
Straughn Jr JM, Richter HE, Conner MG, et al. Predictors of outcome in small cell carcinoma of the cervix: a case series. Gynecol Oncol 2001;83:216–220.
Conner MG, Richter H, Moran CA, et al. Small cell carcinoma of the cervix: a clinicopathologic and immunohistochemical study of 23 cases. Ann Diagn Pathol 2002;6:345–348.
Weed Jr JC, Graff AT, Shoup B, et al. Small cell undifferentiated (neuroendocrine) carcinoma of the uterine cervix. J Am Coll Surg 2003;197:44–51.
Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184–1197.
Barisella M, Andreola S, Rosai J . CD117 in soft tissue sarcomas. Am J Clin Pathol 2002;118:470–471.
Hornick JL, Fletcher CD . Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol 2002;117:188–193.
Lucas DR, Al-Abbadi M, Tabaczka P, et al. c-Kit expression in desmoid fibromatosis: comparative immunohistochemical evaluation of two commercial antibodies. Am J Clin Pathol 2003;119:339–345.
Miettinen M . Are desmoid tumors kit positive [Letter]? Am J Surg Pathol 2001;25:549–550.
Montgomery E, Torbenson MS, Kaushal M, et al. β-Catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol 2002;26:1296–1301.
Yantiss RK, Spiro IJ, Compton CC, et al. Gastrointestinal stromal tumor vs intra-abdominal fibromatosis of the bowel wall: a clinically important differential diagnosis. Am J Surg Pathol 2000;24:947–957.
Abeler VM, Holm R, Nesland JM, et al. Small cell carcinoma of the cervix: a clinicopathologic study of 26 patients. Cancer 1994;73:672–677.
Ambros RA, Park JS, Shah KV, et al. Evaluation of histologic, morphometric, and immunohistochemical criteria in the differential diagnosis of small cell carcinomas of the cervix with particular reference to human papillomavirus types 16 and 18. Mod Pathol 1991;4:586–593.
Masumoto N, Fujii T, Ishikawa M, et al. p16INK4A overexpression and human papillomavirus infection in small cell carcinoma of the uterine cervix. Hum Pathol 2003;34:778–783.
Lizano M, Berumen J, Guido MC, et al. Association between human papillomavirus type 18 variants and histopathology of cervical cancer. J Natl Cancer Inst 1997;89:1227–1231.
Mannion C, Park WS, Man YG, et al. Endocrine tumors of the cervix: morphologic assessment, expression of human papillomavirus, and evaluation for loss of heterozygosity on 1p,3p, 11q, and 17p. Cancer 1998;83:1391–1400.
Stoler MH, Mills SE, Gersell DJ, et al. Small-cell neuroendocrine carcinoma of the cervix: a human papillomavirus type 18-associated cancer. Am J Surg Pathol 1991;15:28–32.
Wistuba II, Thomas B, Behrens C, et al. Molecular abnormalities associated with endocrine tumors of the uterine cervix. Gynecol Oncol 1999;72:3–9.
Cook RM, Miller YE, Bunn Jr PA . Small cell lung cancer: etiology, biology, clinical features, staging, and treatment. Curr Probl Cancer 1993;17:69–141.
Rachtan J . Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer 2002;35:129–136.
Videtic GMM, Stitt LW, Dar AR, et al. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol 2003;21:1544–1549.
Kaye FJ . RB and cyclin dependent kinase pathways: defining a distinction between RB and p16 loss in lung cancer. Oncogene 2002;21:6908–6914.
Junker K, Wiethege T, Müller KM . Pathology of small-cell lung cancer. J Cancer Res Clin Oncol 2000;126:361–368.
Onuki N, Wistuba II, Travis WD, et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999;85:600–607.
Gemba K, Ueoka H, Kiura K, et al. Immunohistochemical detection of mutant p53 protein in small-cell lung cancer: relationship to treatment outcome. Lung Cancer 2000;29:23–31.
Boyer SN, Wazer DE, Band V . E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin–proteasome pathway. Cancer Res 1996;56:4620–4624.
Dyson N, Howley PM, Munger K, et al. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989;243:934–937.
Scheffner M, Huibregtse JM, Vierstra RD, et al. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993;75:495–505.
Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990;63:1129–1136.
Werness BA, Levine AJ, Howley PM . Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990;248:76–79.
Corless CL, McGreevey L, Haley A, et al. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol 2002;160:1567–1572.
Acknowledgements
We thank Ms Prosperidad Amargo for her excellent technical assistance, and Dr Yan Yan for his help with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Lu, D. Overexpression of c-kit protein is an infrequent event in small cell carcinomas of the uterine cervix. Mod Pathol 17, 732–738 (2004). https://doi.org/10.1038/modpathol.3800112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800112
Keywords
This article is cited by
-
HESRG: a novel biomarker for intracranial germinoma and embryonal carcinoma
Journal of Neuro-Oncology (2012)