Abstract
Aneurysmal bone cyst is a benign, cystic lesion of bone composed of blood-filled spaces separated by fibrous septa. Relatively few cases of aneurysmal bone cyst have been cytogenetically characterized, yet abnormalities of the short arm of chromosome 17 appear to be recurrent. In this study, conventional cytogenetic analysis of 43 aneurysmal bone cyst specimens from 38 patients over a 12-year period revealed clonal chromosomal abnormalities in 12 specimens. Karyotypic anomalies of 17p, including a complex translocation and inversion, were identified in eight of these 12 specimens. In an effort to further define the aberrant 17p breakpoint, fluorescence in situ hybridization (FISH) analyses were performed using a series of probe combinations spanning a 5.1 Mb region between the TP53 (17p13.1) and Miller–Dieker lissencephaly syndrome (17p13.3) gene loci. These studies revealed the critical breakpoint locus at 17p13.2, flanked proximally by an RP11-46I8, RP11-333E1, and RP11-457I18 bacterial artificial chromosome (BAC) probe cocktail and distally by an RP11-198F11 and RP11-115H24 BAC and RP5-1050D4 P1 artificial chromosome (PAC) probe cocktail. Overall, abnormalities of the 17p13.2 locus were identified by metaphase and/or interphase cell FISH analysis in 22 of 35 (63%) aneurysmal bone cyst specimens examined including 26 karyotypically normal specimens. These cytogenetic and molecular cytogenetic findings expand our knowledge of chromosomal alterations in aneurysmal bone cyst, further localize the critically involved 17p breakpoint, and provide an alternative approach (ie FISH) for detecting 17p abnormalities in nondividing cells of aneurysmal bone cysts. The latter could potentially be utilized as an adjunct in diagnostically challenging cases.
Similar content being viewed by others
Main
Aneurysmal bone cyst is a benign, often rapidly expanding osteolytic multicystic lesion that was first described by Jaffe and Lichtenstein1 in 1942. Aneurysmal bone cyst most commonly arises in young patients and typically involves the metaphyseal region of long bones and vertebrae.2, 3 Histopathologically, the lesion is composed of varying sized blood-filled spaces separated by connective tissue septa containing bony trabeculae and giant osteoclastic cells.4 Aneurysmal bone cysts are frequently locally aggressive and exhibit a strong tendency to recur.5
Relatively few cases of aneurysmal bone cyst have been cytogenetically characterized.3, 6, 7, 8, 9, 10, 11, 12, 13 Clonal abnormalities of the short arm of chromosome 17 appear to be recurrent and some authors have postulated that alterations of a ‘yet to be determined’ gene on 17p13 may play an important role in the development of aneurysmal bone cyst.3, 7, 8, 9, 10, 12, 13 In this study, the cytogenetic findings of 43 aneurysmal bone cyst samples from 38 patients are reported; several exhibiting novel rearrangements. Moreover, molecular cytogenetic data collected from efforts to further localize the key breakpoint on 17p are described.
Materials and methods
Clinical
Over a 12-year period, representative tissue samples of 43 aneurysmal bone cyst specimens from 38 patients were received for cytogenetic analysis within 36 h of surgery. The clinicopathologic data of all patients are summarized in Table 1. A total of 22 male and 16 female patients with ages ranging from 4 to 48 years were analyzed. In all, 41 of the 43 specimens studied were primary lesions and two were recurrent. Both the biopsy and definitive surgical specimen were available for cytogenetic analysis for four patients. All specimens analyzed were intraosseous and arose de novo with the exception of case 35 that was a secondary aneurysmal bone cyst (associated with a chondroblastoma; also reviewed by Dr K Unni, Mayo Clinic). Excluding case 35, all cases exhibited typical radiographic and microscopic features of aneurysmal bone cyst; that is, lytic, eccentric, expansile masses with well-defined margins histologically composed of blood-filled cystic spaces separated by loose fibroconnective tissue with prominent giant cell reaction and focal reactive bone formation. No solid variant aneurysmal bone cysts were seen.
Cytogenetic Analysis
Cytogenetic analysis was performed on sterile, representative tissue of each case utilizing standard culture and harvest procedures as described previously.14, 15 Briefly, the tissues were disaggregated mechanically and enzymatically and cultured in RPMI 1640 supplemented with 20% fetal bovine serum for 3–8 days. Cells were exposed to an overnight treatment of Colcemid (0.02 μg/ml). Following hypotonic treatment (0.8% sodium citrate for 20 min), the preparations were fixed three times with methanol:glacial acetic acid (3:1). Metaphase cells were banded with Giemsa trypsin. The karyotypes were expressed according to the International System for Human Cytogenetic Nomenclature.16
Probe Design and Development
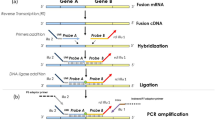
Based on findings from a previous study12 suggesting that the putative 17p13 gene involved in aneurysmal bone cyst was located within the region between the TP53 (17p13.1) and Miller–Dieker lissencephaly syndrome (MDS, 17p13.3) genes, FISH studies were initiated to further define the critically involved breakpoint. This was accomplished by dividing the roughly 5.0 Mb region of interest into 16 segments that sequentially spanned the entire region (Figure 1). Bacterial artificial chromosome (BAC) and P1 artificial chromosome (PAC) probes for each section were identified utilizing the National Center for Biotechnology (NCBI)17 and the Wellcome Trust Sanger Institute Project Ensembl18 databases. Combinations of probe sets were fashioned to flank each defined subregion.
This schematic illustrates the different probe set combinations used to sequentially examine 15 subdivided regions spanning a 5.1 Mb sector flanked by the TP53 (17p13.1) and the MDS (17p13.3) gene loci. The green boxes indicate probe sets labeled in Spectrum Green and the red boxes indicate probe sets labeled in Spectrum Orange. A Spectrum Aqua α-satellite probe for the centromeric region of chromosome 17 was employed for ploidy determination.
Molecular Cytogenetic Analysis (FISH)
Tricolor FISH studies were performed on clonally abnormal metaphase cells [exhibiting the t(16;17) (q22;p13)] and/or cytologic touch preparations of lesional tissue from 35 specimens using BAC and PAC probes (Children's Hospital Oakland Research Institute, Oakland, CA, USA and CTA-D library, Research Genetics, Huntsville, AL, USA) localized to 17p13.1–p13.3 (Figure 1). Probes were nick translated with either Spectrum Green or Spectrum Orange-dUTP utilizing a modification of the manufacturer's protocol (Vysis, Downers Grove, IL, USA). An amount of 1 μg of DNA for each of two or three probes were combined. All nick translation reagents were then multiplied by the total μg of DNA used in the cocktail. Amounts of 200 ng of each probe were hybridized and blocked approximately 15 times with a combination of Human Cot-I DNA (Invitrogen, Carlsbad, CA, USA) and human placental DNA. A spectrum aqua α-satellite probe for the centromeric region of chromosome 17 (Vysis) was employed for ploidy determination.
Prior to hybridization, slides were pretreated at 72°C in 2 × SSC for 2 min and at 37°C in pepsin working solution (20 μl 10% pepsin in 50 ml of 0.1 N HCl) for 3 min. Following pretreatment, the cells and probes were codenaturated at 75°C for 1 min and incubated overnight at 37°C using the HYBrite™ system (Vysis, Downers Grove, IL, USA). Hybridization signals were assessed in 10 metaphase cells or in 200 interphase nuclei with strong, well-delineated signals by two different individuals. An interphase cell specimen was interpreted as abnormal for the 17p13.2 locus if a split of flanking probe signals or loss of the distal flanking probe signal was detected in >10% of the cells evaluated (more than two standard deviations above the average false positive rate). As additional controls, normal peripheral blood lymphocytes, cytologic touch preparations of normal skeletal muscle, and cytologic touch preparations of a unicameral bone cyst were simultaneously hybridized with the same probe sets. Images were acquired using the Cytovision Image Analysis System (Applied Imaging, Santa Clara, CA, USA).
Results
Conventional Cytogenetic Findings
Metaphase cells were obtained in all 43 specimens examined with clonal chromosomal abnormalities detected in 12 (Table 1). Eight specimens exhibited karyotypic anomalies of 17p (cases 15A, 15B, 28, 29, 31, 32, 34, and 37). A balanced 16;17 translocation [t(16;17)(q22;p13)] was observed in three specimens (cases 15A, 15B, and 28, Figure 2). An additional specimen (case 34) showed the 16;17 translocation in the form of a three-way translocation [t(7;17;16)(q21;p13;q22)]. Four additional cases demonstrated 17p13 rearrangements with chromosomal partners other than chromosome 16 (cases 29, 31, 32, and 37, Figure 3). Reciprocal translocations involving chromosomes 1 and 7 were detected in two cases (cases 13 and 24), albeit involving different breakpoints. Moreover, the 1;7 translocation observed in case 24 was shown to be a constitutional anomaly following chromosomal analysis of a peripheral blood sample of this patient.
(a) Representative schematic and GTG-banded partial karyotype of the 16;17 translocation [t(16;17)(q22;p13.2)] observed in case 28. Representative GTG-banded (b) and FISH (c) metaphase cell images of case 28 confirm a split of one of the ‘region seven’ flanking probe sets with translocation of the distal probe set signal (orange) to the der (16) of the t(16;17)(q22;p13). The aqua signals indicate the centromeric regions of the normal and derivative chromosome 17 homologues and the green signals the proximal probe portion of the ‘region seven’ flanking probe set.
(a) Partial GTG-banded karyotype of case 29 illustrating the 6;17 translocation [t(6;17)(p21.2;p13)]. (b) FISH analysis performed on cytologic touch preparations of case 29 showing a representative interphase cell with disruption of ‘region seven’ (single green and single orange signals) and one normal chromosome 17 homologue (juxtaposed orange and green signals). The aqua signals represent the centromeric region of the chromosome 17 homologues. (c) Partial GTG-banded karyotype of case 37 illustrating an inversion of one chromosome 17 homologue (upper and lower arrows indicate breakpoints 17p13 and 17q11.2–12, respectively). (d) FISH analysis performed on cytologic touch preparations of case 37 showing a representative interphase cell with disruption of ‘region seven’ (single green and single orange signals) and one normal chromosome 17 homologue (juxtaposed orange and green signals). The aqua signals represent the centromeric region of the chromosome 17 homologues. (e) Representative GTG-banded karyotype of case 38 showing normal results. (f) FISH analysis performed on cytologic touch preparations of case 38 showing a representative interphase cell with loss of the distal portion of the ‘region seven’ flanking probe set (single green signal without accompanying orange signal) and one normal chromosome 17 (juxtaposed orange and green signals). The aqua signals represent the centromeric region of the chromosome 17 homologues.
Molecular Cytogenetic Findings
Initial FISH studies were performed on cytologic touch preparations of lesional tissue from case 15B characterized by a cytogenetically confirmed t(16;17)(q22;p13) with 15 separate flanking probe combinations covering all 16 segments from the 5.1 Mb region (Figure 1). Only ‘region seven’ (Spectrum Orange-dUTP labeled proximal probes: RP11-46I8, RP11-333E1, RP11-457I8; Spectrum Green-dUTP labeled distal probes: RP11-198F11, RP11-115H24, RP5-1050D4) showed a split of the proximal and distal probe sets indicating a disruption of this 17p13.2 locus. Subsequent FISH studies performed on t(16;17) metaphase cells from case 28 with the ‘region seven’ cocktail probes confirmed signal splitting of this probe set with translocation of the distal probe set to the der(16), (Figure 2). Lastly, the 33 additional aneurysmal bone cyst specimens subjected to FISH with the ‘region seven’ probe set showed the following results: (1) In all, 13 specimens (cases 2, 5, 12B, 16A, 20, 22, 29, 31, 32, 33, 34, 36, and 37) showed a split of one set of probe signals (Figure 3); (2) six specimens (cases 9, 17, 23, 24, 26, and 38) showed loss of the probe set signal distal to ‘region seven’ of the 17p13.2 locus (Figure 3), and; (3) one case demonstrated an extra copy of the probe set signal proximal to ‘region seven’ of the 17p13.2 locus (case 18).
Discussion
Previous cytogenetic studies of aneurysmal bone cyst have revealed 17 cases with clonal chromosomal abnormalities (Table 2),3, 6, 7, 8, 9, 10, 11, 12, 13 Six of these 17 (35%) cases showed a t(16;17)(q22;p13), while seven additional cases demonstrated rearrangement of 17p11–13 with a chromosome partner other than 16q22. Conversely, 16q22 abnormalities exclusive of 17p11–13 abnormalities have been detected in two aneurysmal bone cysts.7, 10 In the current study, three specimens from two patients exhibited an identical t(16;17)(q22;p13) whereas one specimen demonstrated a novel, complex variant translocation, t(7;17;16)(q21;p13;q22). Other anomalies of 17p13 were observed in four additional specimens. Overall, the following chromosomal bands have been detected as 17p11–13 rearrangement partners in aneurysmal bone cyst: 1p34.1–34.3, 2p23, 6p21, 9p22, 14q11.2, 16q22, and 17q12.3, 6, 7, 8, 9, 10, 11, 12, 13 Observation of repeated involvement of 17p11–13 suggests that a putative oncogene important in the pathogenesis of aneurysmal bone cyst lies within this chromosomal region and the manner of its activation is diverse based on the variable 17p chromosomal rearrangement partners.19
In addition to the apparent nonrandom partnership of 16q22 and 17p11–13, the current study reveals two more breakpoints that appear to be recurrently rearranged with 17p13. Winnepenninckx et al12 reported a case of aneurysmal bone cyst in the nose of a 6-year-old girl that exhibited a t(6;17)(p21;p13). Similarly, case 29, an aneurysmal bone cyst arising in the distal fibula of a 13-year-old female, showed an identical 6;17 translocation (Figure 3). Rearrangements of 6p have been seen in several types of tumors of mesenchymal, epithelial, and hematopoietic origin among which are lipoma, uterine leiomyoma, hamartoma, pediatric renal epithelial neoplasm, ovarian carcinoma, and follicular lymphoma.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The putative oncogene, HMGIY (high mobility group protein isoforms I and Y), localized to 6p21 has been proposed to play a role in the development of a number of these neoplasms.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34
An identical t(17;17)(q12;p13) was seen in two previous studies.7, 9 Interestingly, an inversion of chromosome 17 [inv(17)(p13q11.2–12)] involving similar breakpoints was observed in case 37 of the present study (Figure 3). Although rearrangements of the 17q12 band have not been reported as prominent in mesenchymal tumors, the 17q12 locus has received considerable attention because of the localization of the Her-2neu (a.k.a. c-erbB-2, erythroblastic leukemia viral oncogene homolog 2) and RARA (retinoic acid receptor, alpha) genes to this chromosomal band. Assessment of the copy number of the Her-2neu gene and its expression has evolved as an important prognostic indicator in breast, ovarian, and uterine cancers.35, 36, 37, 38, 39 The characteristic rearrangement of RARA with the PML (promyelocytic leukemia) gene on 15q22 has proven to be integral in the diagnosis and treatment of acute promyelocytic leukemia.40, 41, 42
Since the cytogenetic findings of the current study and a review of the literature indicated that the 17p11–13 region likely harbors an oncogene of etiologic importance in aneurysmal bone cyst, efforts to further characterize the critically involved 17p breakpoint were conducted. FISH studies performed on 35 specimens covering a 5.1 Mb region flanked by the TP53 (17p13.1) and MDS (17p13.3) gene loci revealed the key breakpoint lies within a 250 kb region located approximately 5.2–5.5 Mb from the telomere of 17p and bound by RP11-46I8 and RP5-1050D4 at 17p13.2. Approximately 12 genes have been mapped to this 250 kb area.17, 18, 43, 44 Perhaps more specifically, the authors speculate that the exact breakpoint lies within a 15–30 kb region of junctional overlap between BAC probe RP11-46I8 and PAC probe RP5-1050D4, although the relatively gross nature of the approach precludes precise characterization. One gene, FLJ30726, is localized within this smallest region of overlap.44 FLJ30726 is a temporary designation for this gene that encodes for a protein with an unknown function but bears moderate similarity to the zinc-finger protein 35 (ZNF35).44 The ZNF35 protein is believed to be a sequence-specific nucleic acid-binding protein that may function as a transcriptional activator.45, 46
In conclusion, cytogenetic abnormalities of the short arm of chromosome 17 are prominent in aneurysmal bone cyst. Unfortunately, conventional cytogenetic approaches have revealed clonal karyotypic abnormalities in only a fraction of cases examined (12/43 (28%) specimens in the present study), thereby limiting the prospective usefulness of this approach as an adjunct in diagnostically vexing cases. On the other hand, the results of the current study show that FISH probe sets developed to further localize the critical 17p breakpoint, allow detection of 17p13.2 aberrations in a greater subset of aneurysmal bone cysts (22 of 35 (63%) specimens), thereby enhancing the potential diagnostic utility of this assay. Moreover, this FISH approach can also be performed on nondividing cells of aneurysmal bone cysts. Lastly, the more precise localization of the critical 17p breakpoint in this study should provide direction for future molecular studies aimed at deciphering the underlying gene that may prove central to aneurysmal bone cyst development.
References
Jaffe HL, Lichtenstein L . Solitary unicameral bone cyst with emphasis on the roentgen picture, the pathologic appearances and the pathogenesis. Arch Surg 1942;44:1004–1025.
Martinez V, Sissons HA . Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer 1988;61:2291–2304.
Sciot R, Dorfman H, Brys P, et al. Cytogenetic–morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions. A report from the CHAMP study group. Mod Pathol 2000;13:1206–1210.
Biesecker JL, Marcove RC, Huvos AG, et al. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer 1970;26:615–625.
Dorfman HD, Czeniak B . Giant cell lesions. In: Bone Tumors, Mosby CV, Inc.: St. Louis, 1998.
Baruffi MR, Neto JB, Barbieri CH, et al. Aneurysmal bone cyst with chromosomal changes involving 7q and 16p. Cancer Genet Cytogenet 2001;129:177–180.
Dal Cin P, Kozakewich HP, Goumnerova L, et al. Variant translocations involving 16q22 and 17p13 in solid variant and extraosseous forms of aneurysmal bone cyst. Genes Chromosomes Cancer 2000;28:233–234.
Herens C, Thirty A, Dresse MF, et al. Translocation (16;17)(q22;p13) is a recurrent anomaly of aneurysmal bone cysts. Cancer Genet Cytogenet 2001;127:83–84.
Nielsen GP, Fletcher CDM, Smith MA, et al. Soft tissue aneurysmal bone cyst: a clinicopathologic study of five cases. Am J Surg Pathol 2002;26:64–69.
Panoutsakopoulos G, Pandis N, Kyriazoglou I, et al. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer 1999;26:265–266.
Pfeifer FM, Bridge JA, Neff JR, et al. Cytogenetic findings in aneurysmal bone cysts. Genes Chromosomes Cancer 1991;3:416–419.
Winnepenninckx V, Debiec-Rychter M, Jorissen M, et al. Aneurysmal bone cyst of the nose with 17p13 involvement. Virchows Arch 2001;439:636–639.
Wyatt-Ashmead J, Bao L, Eilert RE, et al. Primary aneurysmal bone cysts: 16q22 and/or 17p13 chromosome abnormalities. Pediatr Dev Pathol 2001;4:418–419.
Nelson M, Perry D, Ginsburg G, et al. Translocation (1;4)(p31;q34) in nonossifying fibroma. Cancer Genet Cytogenet 2003;142:142–144.
Safar A, Nelson M, Neff JR, et al. Recurrent anomalies of 6q25 in chondromyxoid fibroma. Hum Pathol 2000;31:306–311.
Helman F, (ed). ISCN: An International System for Human Cytogenetic Nomenclature. CT, S. Karger Publishers, Inc.: Farmington, 1995.
National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov. 2003.
The Wellcome Trust Sanger Institute Project Ensembl. http://www.ensembl.org. 2003.
Rosenberg AE, Nielsen GP, Fletcher JA . Aneurysmal bone cyst. In: Fletcher CDM, Unni KK, Mertens F (eds). WHO Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 338–339.
Argani P, Hawkins A, Griffin CA, et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;q12) chromosome translocation. Am J Pathol 2001;158:2089–2096.
Dal Cin P, Wanschura S, Christiaens MR, et al. Hamartoma of the breast with involvement of 6p21 and rearrangement of HMGIY. Genes Chromosomes Cancer 1997;20:90–92.
Kazmierczak B, Dal Cin P, Wanschura S, et al. HMGIY is the target of 6p21.3 rearrangements in various benign mesenchymal tumors. Genes Chromosomes Cancer 1998;23:279–285.
Kazmierczak B, Meyer-Bolte K, Tran KH, et al. A high frequency of tumors with rearrangements of genes of the HMGI(Y) family in a series of 191 pulmonary chondroid hamartomas. Genes Chromosomes Cancer 1999;26:125–133.
Ligon AH, Morton CC . Genetics of uterine leiomyomata. Genes Chromosomes Cancer 2000;28:235–245.
Mandahl N, Hoglund M, Mertens F, et al. Cytogenetic aberrations in 188 benign and borderline adipose tissue tumors. Genes Chromosomes Cancer 1994;9:207–215.
Miura I, Ohshima A, Takahashi N, et al. A new non-random chromosomal translocation t(3;6)(q27;p21.3) associated with BCL6 rearrangement in two patients with non-Hodgkin's lymphoma. Int J Hematol 1996;64:249–256.
Ohno H, Kerckaert JP, Bastard C, et al. Heterogeneity in B-cell neoplasms associated with rearrangement of the LAZ3 gene on chromosome band 3q27. Jpn J Cancer Res 1994;85:592–600.
Pejovic T, Heim S, Mandahl N, et al. Chromosome aberrations in 35 primary ovarian carcinomas. Genes Chromosomes Cancer 1992;4:58–68.
Tallini G, Dal Cin P, Rhoden KJ, et al. Expression of HMGI-C and HMGI(Y) in ordinary lipoma and atypical lipomatous tumors: immunohistochemical reactivity correlates with karyotypic alterations. Am J Pathol 1997;151:37–43.
Tallini G, Vanni R, Manifioletti G, et al. HMGI-C and HMGI(Y) immunoreactivity correlates with cytogenetic abnormalities in lipomas, pulmonary chondroid hamartomas, endometrial polyps, and uterine leiomyomas and is compatible with rearrangement of the HMGI-C and HMGI(Y) genes. Lab Invest 2000;80:359–369.
Thompson FH, Emerson J, Alberts D, et al. Clonal chromosome abnormalities in 54 cases of ovarian carcinoma. Cancer Genet Cytogenet 1994;73:33–45.
Xiao S, Lux ML, Reeves R, et al. HMGI(Y) activation by chromosome 6p21 rearrangements in multilineage mesenchymal cells from pulmonary hamartoma. Am J Pathol 1997;150:901–910.
Friedmann M, Holth LT, Zoghbi HY, et al. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res 1993;21:4259–4267.
Wood LJ, Mukherjee M, Dolde CE, et al. HMG-I/Y, a new c-Myc target gene and potential oncogene. Mol Cell Biol 2000;20:5490–5502.
Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res 1990;50:4332–4337.
Gullick WJ, Love SB, Wright C, et al. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br J Cancer 1991;63:434–438.
Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992;10:1049–1056.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–182.
Tandon AK, Clark GM, Chamness GC, et al. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 1989;7:1120–1128.
Grimwade D, Lo Coco F . Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia 2002;16:1959–1973.
Mistry AR, Pedersen EW, Solomon E, et al. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev 2003;17:71–97.
Rowley JD, Golomb HM, Dougherty C . 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet 1977;1:549–550.
Cancer Genome Anatomy Project. http://cgap.nci.nih.gov. 2003.
Weitzmann Institute of Science. http://bioinformatics.weizmann.ac.il. 2003.
Lanfrancone L, Pengue G, Pandolfi PP, et al. Structural and functional organization of the HF.10 human zinc finger gene (ZNF35) located on chromosome 3p21–p22. Genomics 1992;12:720–728.
Pengue G, Cannada-Bartoli P, Lania L . The ZNF35 human zinc finger gene encodes a sequence-specific DNA-binding protein. FEBS Lett 1993;321:233–236.
Acknowledgements
This work was supported in part by the John A Wiebe, Jr, Children's Health Care Fund, Nebraska State Department of Health LB595, and NIH (P30 CA 36727). We thank Ms. Kimberly Christian for her expert secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Althof, P., Ohmori, K., Zhou, M. et al. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol 17, 518–525 (2004). https://doi.org/10.1038/modpathol.3800090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800090
Keywords
This article is cited by
-
Morphologisches Spektrum USP6-rearrangierter Läsionen
Der Pathologe (2018)
-
Treatment of aneurysmal bone cysts by percutaneous CT-guided injection of calcitonin and steroid
Skeletal Radiology (2017)
-
Soft-tissue aneurysmal bone cyst with translocation t(17;17)(p13;q21) corresponding to COL1A1 and USP6 loci
Skeletal Radiology (2015)
-
Response of an aggressive periosteal aneurysmal bone cyst (ABC) of the radius to denosumab therapy
World Journal of Surgical Oncology (2014)
-
Solid variant of aneurysmal bone cyst in the acetabulum
European Orthopaedics and Traumatology (2013)