Abstract
Microsatellite instability and loss of heterozygosity has been implicated in ovarian carcinogenesis. The reported frequency of microsatellite instability in human ovarian cancer varies significantly owing to the use of heterogeneous tumor histotypes and various microsatellite markers in different laboratories. In this study, we determined the frequency of microsatellite instability in 74 ovarian endometrioid carcinomas using four microsatellite markers (BAT25, BAT26, D5S346, D17S250), and examined hMLH1 and hMSH2 protein expression. In all, 20% of the tumors were microsatellite instability high (two or more markers showing instability) and 12% were microsatellite instability low (one marker showed instability). Loss of hMLH1 and/or hMSH2 expression was found in nine of 15 microsatellite instability-high tumors. The microsatellite instability-high phenotype tended to occur more frequently in low-grade tumors (P=0.053), but did not correlate with clinical stage. Totally, 38% of cases also displayed loss of heterozygosity at D17S250; this loss of heterozygosity was associated with high clinical stage (P=0.097). Our results indicate that both microsatellite and loss of heterozygosity at D17S250 are involved in the development of ovarian endometrioid carcinoma.
Similar content being viewed by others
Main
The genetic pathways involved in the development of human ovarian cancer are poorly defined because of the lack of morphologically well-defined precursor lesions and the many different histologic subtypes. Both microsatellite instability and loss of heterozygosity have been implicated in ovarian carcinogenesis. Microsatellite instability results from the inactivation of genes involved in DNA mismatch repair. Defective DNA mismatch repair gene function is thought to promote tumorigenesis by accelerating mutations in oncogenes and tumor suppressor genes. Microsatellite instability is commonly seen in hereditary nonpolyposis colorectal cancer syndrome.1 This is caused by loss of function of DNA mismatch genes such as hMLH1, hMSH2, hPMS1, hPMS2, hMSH3, and hMSH6.1 Loss of expression of either hMLH1 or hMSH2 has been described in these tumors and contributed to the development of most microsatellite instability phenotypes.2, 3, 4, 5
The reported frequency of microsatellite instability in ovarian tumors varies, ranging from 0 to 50%.6, 7, 8, 9, 10, 11, 12 In large part, this large variation is due to the use of different microsatellite markers and different criteria for defining tumors as microsatellite instability-positive. As serous carcinoma is the most common epithelial ovarian cancer, most microsatellite-instability studies have focused on this subtype. The studies often included endometrioid, mucinous, and clear-cell carcinomas and the results were reported together.6, 7, 9, 13, 14, 15 Consequently, the differences in the morphology, etiology, and clinical behavior between these ovarian epithelial tumors have made interpretation of these previous studies difficult.
To circumvent these limitations, our studies were performed using a panel of markers (BAT25, BAT26, D5S346, and D17S250) to study a homogeneous group of ovarian endometrioid carcinomas. These tumors were chosen because of their shared characteristics with endometrial endometrioid carcinoma, a type of tumor known to have microsatellite instability. The goals of this study were to: (1) determine the frequency of microsatellite instability in ovarian endometrioid carcinoma; (2) examine the loss of expression of the hMLH1 and hMSH2 proteins in both microsatellite instability-positive and microsatellite stable tumors, and (3) correlate microsatellite with clinical variables, such as tumor grade and clinical stage.
Materials and methods
Matched pairs of formalin-fixed, paraffin-embedded normal and tumor tissue specimens were obtained from 74 patients. The pathologic characteristics of each tumor were confirmed by a pathologist (either JL or WZ). For the tumor with focal serous carcinoma, only endometrioid components were dissected and DNAs extracted for further analysis. The tumor was microdissected from adjacent normal tissue with an 18-gauge needle under light microscopy. DNA from each sample was extracted and analyzed for microsatellite instability using the panel of four microsatellite markers recommended by the National Cancer Institute:16, 17 BAT25, BAT26, D5S346, and D17S250 using multiplex polymerase chain reaction. A fifth marker, D2S123, was also included but not consistently amplified in most of cases and therefore was not included in the final analysis. Oligonucleotide primers were fluorescent-labeled as previously described.18, 19 Multiplex polymerase chain reaction was performed in a 15 μl volume containing 40 ng of DNA, 5 pmol of fluorescent-labeled primers (Life Technologies, Gaithersburg, MD, USA), 2.5 mM MgCl2, 200 μM dNTPs, and 2 U AmpliTaq Gold DNA polymerase (Applied Biosystems). The reaction was denatured at 95°C for 6 min, 54°C for 30 s, and 72°C for 40 s; and extension at 72°C for 10 min. The fluorescent-labeled products were analyzed by capillary electrophoresis on an ABI 3700 DNA Analyzer using GeneScan analysis software (Applied Biosystems). Tumors in which only 1 marker showed instability were defined as microsatellite instability-low. Tumors in which no markers exhibited microsatellite instability were defined as microsatellite stable. Loss of heterozygosity was also defined as an identical pattern in one of two alleles, but complete loss in the second allele and scored in BAT25, BAT26, D5S346, and D17S250.
Immunohistochemical staining was carried out by incubating tissue sections with either a 1 : 30 dilution of purified mouse anti-hMLH1 antibody (PharMingen International, San Diego, CA, USA) or a 1 : 100 dilution of mouse monoclonal antibody to hMSH2 (Ab-2, Oncogene, La Jolla, CA, USA). Immunoreactive proteins were visualized using EnVision-horseradish peroxidase kit (Dako, Carpinteria, CA, USA) for hMLH1 and LSAB2 horseradish peroxidase kit (Dako) for hMSH2. Clinical information was obtained from pathology reports, patient charts, or both. Statistical analyses of microsatellite instability status, loss of heterozygosity, and immuno-histochemical data were performed using the χ2 analysis. A P-value <0.05 was considered to be statistically significant.
Results
Microsatellite Instability in Ovarian Endometrioid Carcinomas
Of the 74 tumors, 15 (20%) showed allelic shifts in two or more loci and were designated microsatellite instability-high. One representative tumor of microsatellite instabilities high is shown in Figure 1. Nine tumors (12%) demonstrated an allelic shift in only one locus and were designated microsatellite instability-low. The remaining 50 cases showed no changes in any of the loci that were designated as microsatellite stable. We wanted to determine whether the microsatellite status of ovarian endometrioid carcinomas correlates with clinicopathologic features. Our results showed that the microsatellite status of ovarian endometrioid carcinomas did not correlate with clinical stage of the disease (Table 1), although high-grade ovarian endometrioid carcinomas tended to have a microsatellite stable phenotype, and the low-grade ovarian endometrioid carcinomas tended to have a microsatellite instability-high phenotype (Table 1; P=0.053).
hMLH1 and hMSH2 Protein Expression in Ovarian Endometrioid Carcinoma
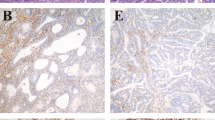
Microsatellite instability is associated with mutations of DNA mismatch repair genes, especially hMLH1 and hMSH2.2, 3, 5 To determine expression levels of the DNA mismatch repair proteins, immuno-histochemical analysis was performed. hMLH1 and hMSH2 proteins were present in the majority (89–100%) of microsatellite stable and microsatellite instability-low tumors (Table 2). In contrast, loss of either hMLH1 or hMSH2 expression occurred in a total of nine (60%) of 15 microsatellite instability-high tumors. Representative photomicrographs are shown in Figure 2.
Immunohistochemical expression of hMLH1 and hMSH2 proteins in ovarian endometrioid carcinoma. A, C, and E, stained with anti-hMLH1; B, D, and F, stained with anti-hMSH2. Immunoreactive proteins were visualized using EnVision-horseradish peroxidase kit for hMLH1 and LSAB2 horseradish peroxidase kit for hMSH2. Magnification is × 100.
Loss of Heterozygosity in Ovarian Endometrioid Carcinoma
In all, 74 cases of ovarian endometrioid carcinoma were analyzed for loss of heterozygosity in markers D5S346 and D17S250. Loss of heterozygosity occurred in 32 (43%) of 74 ovarian endometrioid carcinoma, nine of these showed loss of heterozygosity at D5S346, and 26 showed loss of heterozygosity at D17S250. A representative image is shown in Figure 3. Of these 32 tumors with loss of heterozygosity, 24 were microsatellite stable, three were microsatellite instability-low, and five were microsatellite-high. Loss of heterozygosity at D17S250 correlated with high clinical stage (P=0.097) (Table 3).
Analysis of loss of heterozygosity at the following loci: BAT25, BAT26, D5S246, and D17S250 in a representative tumor. For each marker, the top graph represents normal DNA and the bottom graph represents tumor DNA. In this case, the tumor showed loss of heterozygosity in D17S250 as indicated by an arrow.
Discussion
Our results demonstrate that a significant number (20%) of ovarian endometrioid carcinoma are microsatellite instability-high. Analysis of clinical characteristics of ovarian endometrioid caricnoma indicated that microsatellite instability-positive tumors tended to be low-grade, whereas tumor with loss of heterozygosity tended to be a higher grade and clinical stage. These results suggest that microsatellite instability plays an important role in the pathogenesis of sporadic ovarian endometrioid carcinoma. Our results further show that 60% of the microsatellite instability-high tumors have loss of expression of the DNA mismatch repair gene, hMLH1 or hMSH2. This indicates that immuno-histochemical staining for the corresponding DNA mismatch repair proteins could help identify ovarian endometrioid carcinomas with the microsatellite instability phenotype. These features are similar to those observed in microsatellite instability positive sporadic tumors of the colon and endometrium, supporting a similar role of microsatellite instability in the pathogenesis of tumors in these organs.3, 20
Most studies of microsatellite instability in ovarian cancer were performed before the use of a standard panel of microsatellite markers and standardized criteria for determining microsatellite instability. Some studies classified tumors as microsatellite instability if any allelic shift was detected, whereas the National Cancer Institute recommended alterations in at least two markers.16, 17 This would explain the higher frequency of microsatellite instability observed in some of the earlier studies. Application of the National Cancer Institute criteria to some of the previous studies was possible and indicated that microsatellite instability was more frequent in ovarian endometrioid carcinomas than in serous ovarian carcinomas.9, 11, 16 This was consistent with studies of the endometrium, in which microsatellite instability was also infrequent in serous carcinomas.21, 22, 23, 24
Immunohistochemical staining for hMLH1 and hMSH2 proteins showed microsatellite instability-high tumors in 60% of cases. Only one microsatellite instability stable tumor showed loss of hMSH2 expression (2%). These results indicated that immunohistochemical analysis for hMLH1 and hMSH2 was highly specific for detecting microsatellite instability. This is consistent with other reports demonstrating a correlation between loss of DNA mismatch repair gene function and the microsatellite instability phenotype.2, 3, 4, 5 Similarly, other studies have also shown a more frequent loss of hMLH1 expression compared with loss of hMSH2 expression.2, 3, 5, 25 Despite the importance of hMLH1 in microsatellite instability, the presence of normal hMLH1 expression in 53% of the microsatellite instability-high tumors in our study suggested the involvement of other DNA mismatch repair genes as well, such as hPMS1, hPMS2, hMSH3, and hMSH6.1 A major mechanism for loss of hMLH1 expression in sporadic colon and endometrial cancers was hypermethylation of the hMLH1 promoter, resulting in silencing of the hMLH1 gene.26, 27 It will be interesting to determine whether this holds true for ovarian endometrioid carcinoma. Gras et al8 have already begun to address this question; their studies describe hMLH1 methylation in ovarian endometrioid carcinoma with microsatellite instability.
We found a high frequency of loss of heterozygosity in ovarian endometrioid carcinoma using the same markers that we used to detect microsatellite instability. In most of these cases (81%), loss of heterozygosity involved the D17S250 marker, a DNA region corresponding to the long arm of chromosome 17 (17q11.2–q12). Loss of heterozygosity in chromosome 17 has been reported in epithelial ovarian tumors, including serous, endometrioid, mucinous, and clear-cell carcinomas.11, 28, 29, 30, 31, 32, 33, 34, 35, 36 However, loss of heterozygosity in 17q does not appear to be unique to ovarian tumors. Indeed, it has also been described in several other types of cancers, although it is neither a prominent nor a consistent alteration.20, 24, 37, 38, 39, 40 In general, chromosomal instability in tumors as assessed by loss of heterozygosity was associated with advanced-stage disease.11, 29 This was consistent with our results that showed an association between loss of heterozygosity and late-stage tumors. Although the pertinent gene is not known, the prevalence of loss of heterozygosity in the D17S250 region in ovarian endometrioid carcinoma suggested that this region may harbor a tumor suppressor gene involved in tumor progression.
In conclusion, our results indicate that both microsatellite and loss of heterozygosity in the D17S250 region may play a significant role in ovarian endometrioid carcinoma. These two pathways do not appear to be mutually exclusive, since one-third of the microsatellite instability-high tumors in our study also demonstrated loss of heterozygosity in the D17S250 region. This suggested that microsatellite instability and loss of heterozygosity are distinct, but not necessarily independent pathways in the pathogenesis of ovarian endometrioid carcinoma. In addition, immuno-histochemical stainings for hMLH1 and hMSH2 are highly specific in identifying these tumors with a microsatellite-instability phenotype.
References
Loukola A, Eklin K, Laiho P, et al. Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res 2001;61:4545–4549.
Chaves P, Cruz C, Lage P, et al. Immunohistochemical detection of mismatch repair gene proteins as a useful tool for the identification of colorectal carcinoma with the mutator phenotype. J Pathol 2000;191:355–360.
Chiaravalli AM, Furlan D, Facco C, et al. Immunohistochemical pattern of hMSH2/hMLH1 in familial and sporadic colorectal, gastric, endometrial and ovarian carcinomas with instability in microsatellite sequences. Virchows Arch 2001;438:39–48.
Ichikawa Y, Lemon SJ, Wang S, et al. Microsatellite instability and expression of MLH1 and MSH2 in normal and malignant endometrial and ovarian epithelium in hereditary nonpolyposis colorectal cancer family members. Cancer Genet Cytogenet 1999;112:2–8.
Parc YR, Halling KC, Wang L, et al. HMSH6 alterations in patients with microsatellite instability-low colorectal cancer. Cancer Res 2000;60:2225–2231.
Fujita M, Enomoto T, Yoshino K, et al. Microsatellite instability and alterations in the hMSH2 gene in human ovarian cancer. Int J Cancer 1995;64:361–366.
Allen HJ, DiCioccio RA, Hohmann P, et al. Microsatellite instability in ovarian and other pelvic carcinomas. Cancer Genet Cytogenet 2000;117:163–166.
Gras E, Catasus L, Arguelles R, et al. Microsatellite instability, MLH-1 promoter hypermethylation, and frameshift mutations at coding mononucleotide repeat microsatellites in ovarian tumors. Cancer 2001;92:2829–2836.
King BL, Carcangiu ML, Carter D, et al. Microsatellite instability in ovarian neoplasms. Br J Cancer 1995;72:376–382.
Park TW, Felix JC, Wright Jr TC . X chromosome inactivation and microsatellite instability in early and advanced bilateral ovarian carcinomas. Cancer Res 1995;55:4793–4796.
Pieretti M, Cavalieri C, Conway PS, et al. Genetic alterations distinguish different types of ovarian tumors. Int J Cancer 1995;64:434–440.
Suzuki K, Dai T, Suzuki I, et al. Low mutation incidence in polymorphic noncoding short mononucleotide repeats in gastrointestinal cancer of the microsatellite mutator phenotype pathway. Cancer Res 2002;62:1961–1965.
Arzimanoglou II, Lallas T, Osborne M, et al. Microsatellite instability differences between familial and sporadic ovarian cancers. Carcinogenesis 1996;17:1799–1804.
Haas CJ, Diebold J, Hirschmann A, et al. Microsatellite analysis in serous tumors of the ovary. Int J Gynecol Pathol 1999;18:158–162.
Tangir J, Loughridge NS, Berkowitz RS, et al. Frequent microsatellite instability in epithelial borderline ovarian tumors. Cancer Res 1996;56:2501–2505.
Sood AK, Holmes R, Hendrix MJ, et al. Application of the National Cancer Institute international criteria for determination of microsatellite instability in ovarian cancer. Cancer Res 2001;61:4371–4374.
Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–5257.
Kabbani W, Houlihan PS, Luthra R, et al. Mucinous and nonmucinous appendiceal adenocarcinomas: different clinicopathological features but similar genetic alterations. Mod Pathol 2002;15:599–605.
Lee JH, Abraham SC, Kim HS, et al. Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol 2002;161:611–618.
Toda T, Oku H, Khaskhely NM, et al. Analysis of microsatellite instability and loss of heterozygosity in uterine endometrial adenocarcinoma. Cancer Genet Cytogenet 2001;126:120–127.
Caduff RF, Johnston CM, Svoboda-Newman SM, et al. Clinical and pathological significance of microsatellite instability in sporadic endometrial carcinoma. Am J Pathol 1996;148:1671–1678.
Catasus L, Matias-Guiu X, Machin P, et al. BAX somatic frameshift mutations in endometrioid adenocarcinomas of the endometrium: evidence for a tumor progression role in endometrial carcinomas with microsatellite instability. Lab Invest 1998;78:1439–1444.
Lax SF, Kendall B, Tashiro H, et al. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 2000;88:814–824.
Pere H, Tapper J, Wahlstrom T, et al. Distinct chromosomal imbalances in uterine serous and endometrioid carcinomas. Cancer Res 1998;58:892–895.
Peiro G, Diebold J, Mayr D, et al. Prognostic relevance of hMLH1, hMSH2, and BAX protein expression in endometrial carcinoma. Mod Pathol 2001;14:777–783.
Ellenson LH . hMLH1 promoter hypermethylation in microsatellite instability-positive endometrial carcinoma. Cause or consequence? Am J Pathol 1999;155:1399–1402.
Esteller M, Catasus L, Matias-Guiu X . hMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Am J Pathol 1999;155:1767–1772.
Osborne RJ, Leech V . Polymerase chain reaction allelotyping of human ovarian cancer. Br J Cancer 1994;69:429–438.
Shenson D, Gallion H, Powell D, et al. Loss of heterozygosity and genomic instability in synchronous endometrioid tumors of the ovary and endometrium. Cancer 1995;76:650–657.
Wertheim I, Tangir J, Muto MG, et al. Loss of heterozygosity of chromosome 17 in human borderline and invasive epithelial ovarian tumors. Oncogene 1996;12:2147–2153.
Garcia A, Bussaglia E, Machin P, et al. Loss of heterozygosity on chromosome 17q in epithelial ovarian tumors: association with carcinomas with serous differentiation. Int J Gynecol Pathol 2000;19:152–157.
Eccles DM, Cranston G, Steel CM, et al. Allele losses on chromosome 17 in human epithelial ovarian carcinoma. Oncogene 1990;5:1599–1601.
Dodson MK, Hartmann LC, Cliby WA, et al. Comparison of loss of heterozygosity patterns in invasive low-grade and high-grade epithelial ovarian carcinomas. Cancer Res 1993;53:4456–4460.
Phillips N, Ziegler M, Saha B, et al. Allelic loss on chromosome 17 in human ovarian cancer. Int J Cancer 1993;54:85–91.
Russell SE, Hickey GI, Lowry WS, et al. Allele loss from chromosome 17 in ovarian cancer. Oncogene 1990;5:1581–1583.
Sato T, Saito H, Morita R, et al. Allelotype of human ovarian cancer. Cancer Res 1991;51:5118–5122.
Sonoda G, du Manoir S, Godwin AK, et al. Detection of DNA gains and losses in primary endometrial carcinomas by comparative genomic hybridization. Genes Chromosomes Cancer 1997;18:115–125.
Wong MP, Lam WK, Wang E, et al. Primary adenocarcinomas of the lung in nonsmokers show a distinct pattern of allelic imbalance. Cancer Res 2002;62:4464–4468.
Koga T, Iwasaki H, Ishiguro M, et al. Frequent genomic imbalances in chromosomes 17, 19, and 22q in peripheral nerve sheath tumours detected by comparative genomic hybridization analysis. J Pathol 2002;197:98–107.
Varis A, Wolf M, Monni O, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res 2002;62:2625–2629.
Acknowledgements
This work was supported in part by Grant P01CA64602-1 from the National Cancer Institute, Institutional start-up funds, and an Institutional Research Grant and Career Development Award from MD Anderson Cancer Center SPORE on Ovarian cancer to JL. We thank Ms Valerie Dunmire and Jennifer Merino for technical assistance, Ms Carmen Salazar for imaging, and Drs Atac Baykal and Kathy Qi Cai for critical reading of the manuscript and statistical analysis. We also thank Ms Mariann Crapanzano for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Albarracin, C., Chang, KH. et al. Microsatellite instability and expression of hMLH1 and hMSH2 proteins in ovarian endometrioid cancer. Mod Pathol 17, 75–80 (2004). https://doi.org/10.1038/modpathol.3800017
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800017
This article is cited by
-
Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome
Nature Communications (2020)
-
Pathogenesis of the Endometriosis-Related Ovarian Neoplasms
Current Obstetrics and Gynecology Reports (2014)
-
Evaluating cell lines as tumour models by comparison of genomic profiles
Nature Communications (2013)
-
Lynch syndrome-associated neoplasms: a discussion on histopathology and immunohistochemistry
Familial Cancer (2013)
-
Mismatch repair and treatment resistance in ovarian cancer
BMC Cancer (2006)