Abstract
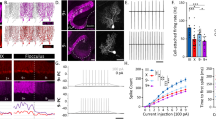
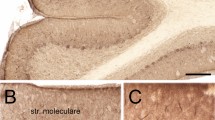
NEURONS contain distinct compartments including dendrites, dendritic spines, axons and synaptic terminals1. The molecular mechanisms that generate and distinguish these compartments, although largely unknown, may involve the small GTPases Rac and Cdc42 (ref. 2), which appear to regulate actin polymerization3. Having shown that perturbations of Racl activity block the growth of axons but not dendrites of Drosophila neurons2, we investigated whether this also applies to mammals by examining transgenic mice expressing constitutively active human Racl in Purkinje cells. We found that these mice were ataxic and had a reduction of Purkinje-cell axon terminals in the deep cerebellar nuclei, whereas the dendritic trees grew to normal height and branched extensively. Unexpectedly, the dendritic spines of Purkinje cells in developing and mature cerebella were much reduced in size but increased in number. These 'mini' spines often form supernumerary synapses. These differential effects of perturbing Racl activity indicate that there may be distinct mechanisms for the elaboration of axons, dendrites and dendritic spines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cajal, S. R. Histology of the Nervous System of Man and Vertebrates (Oxford Univ. Press, 1995).
Luo, L., Liao, Y. J., Jan, L. Y. & Jan, Y. N. Genes Dev. 8, 1787–1802 (1994).
Hall, A. A. Rev. Cell Biol. 10, 31–54 (1994).
Palay, S. L. & Chan-Palay, V. Cerebellar Cortex: Cytology and Organization (Springer, New York, 1974).
Ito, M. The Cerebellum and Neural Control (Raven, New York, 1984).
Oberdick, J. et al. Neuron 10, 1007–1018 (1993).
Smeyne, R. J. et al. Molec. cell. Neurosci. 6, 230–251 (1995).
Dunham, N. W. & Miya, T. S. J. Am. Pharmac. Assoc. 46, 208–209 (1957).
Jande, S. S., Maler, L. & Lawson, D. E. M. Nature 294, 765–767 (1981).
Mignery, G. A., Südhof, T. C., Takei, K. & De Camilli, P. Nature 342, 192–195 (1989).
Jahn, R., Schiebler, R., Ouimet, C. & Greengard, P. Proc. natn. Acad. Sci. U.S.A. 82, 4137–4141 (1985).
Sotelo, C. J. Neurocytol. 19, 737–755 (1990).
Dusart, I. & Sotelo, C. J. comp. Neurol. 347, 211–232 (1994).
Altman, J. J. comp. Neurol. 145, 399–464 (1972).
Berry, M. & Bradley, P. Brain Res. 112, 1–35 (1976).
Harris, K. M. & Stevens, J. K. J. Neurosci. 8, 4455–4469 (1988).
Vojtek, A. B. & Cooper, J. A. Cell 82, 527–529 (1995).
Harris, K. W. & Kater, S. B. A. Rev. Neurosci. 17, 341–371 (1994).
Rakic, P. & Sidman, R. L. J. comp. Neurol. 152, 133–162 (1973).
Baptista, C. A., Hatten, M. E., Blazeski, R. & Mason, C. A. Neuron 12, 243–260 (1994).
Didsbury, J., Weber, R. F., Bokoch, G. M., Evans, T. & Snyderman, R. J. biol. Chem. 264, 16378–16382 (1989).
Wood, T. L. et al. DNA 7, 585–593 (1988).
Moll, J., Sansig, G., Fattori, E. & van der Putten, H. Oncogene 6, 863–866 (1991).
Hogan, B., Costantini, F. & Lacy, E. Manipulating the Mouse Embryo (Cold Spring Harbor Laboratory, Cold Spring Harbor, 1986).
Wisden, W. & Morris, B. J. in In Situ Hybridization Protocols for the Brain (eds Widsen, W. & Morris, B. J.) 9–34 (Academic, London, 1994).
Jones, K. R., Farinas, I., Backus, C. & Reichardt, L. F. Cell 76, 989–999 (1994).
Guenet, J.-L., Sotelo, C. & Mariani, J. J. Hered. 74, 105–108 (1983).
Roffler-Tarlov, S., Beart, P. M., O'Gorman, S. & Sidman, R. L. Brain Res. 168, 75–95 (1979).
Ajima, A., Hensch, T., Kado, R. T. & Ito, M. Neurosci. Res. 12, 281–286 (1991).
Fairen, A. & Smith-Fernandez, A. Micros. Res. Tech. 23, 289–305 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luo, L., Hensch, T., Ackerman, L. et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379, 837–840 (1996). https://doi.org/10.1038/379837a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/379837a0
This article is cited by
-
Incorporating neuro-inspired adaptability for continual learning in artificial intelligence
Nature Machine Intelligence (2023)
-
Rac1 GTPase activation impairs fear conditioning-induced structural changes in basolateral amygdala neurons and long-term fear memory formation
Neuropsychopharmacology (2023)
-
CYFIP1 overexpression increases fear response in mice but does not affect social or repetitive behavioral phenotypes
Molecular Autism (2019)
-
Rac1 Modulates Excitatory Synaptic Transmission in Mouse Retinal Ganglion Cells
Neuroscience Bulletin (2019)
-
P-Rex1 Overexpression Results in Aberrant Neuronal Polarity and Psychosis-Related Behaviors
Neuroscience Bulletin (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.