Abstract
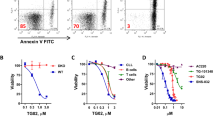
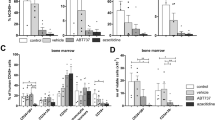
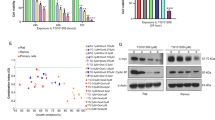
ACUTE lymphoblastic leukaemia (ALL) is the most common cancer of childhood. Despite the progress achieved in its treatment, 20% of cases relapse and no longer respond to chemotherapy. The most common phenotype of ALL cells share surface antigens with very early precursors of B cells and are therefore believed to originate from this lineage1,3Characterization of the growth requirement of ALL cells indicated that they were dependent on various cytokines, suggesting paracrine and/or autocrine growth regulation4–6. Because many cytokines induce tyrosine phosphorylation in lymphoid progenitor cells, and constitutive tyrosine phosphorylation is commonly observed in B-lineage leukaemias7,8, attempts have been made to develop protein tyrosine kinase (PTK) blockers of leukaemia cell growth9,10. Here we show that leukaemic cells from patients in relapse have con-stitutively activated Jak-2 PTK. Inhibition of Jak-2 activity by a specific tyrosine kinase blocker, AG-490, selectively blocks leukaemic cell growthin vitro and in vivo by inducing programmed cell death, with no deleterious effect on normal haematopoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nadler, L. M. et al. J. Immun. 131, 244–250 (1983).
Greaves, M. F. Science 234, 697–704 (1986).
Uckun, F. M. et al. New Engl. J. Med. 329, 1296–1301 (1993).
Williams, G. T., Smith, C. A., Spooncer, E., Dexter, T. M. & Jaylor, D. R. Nature 343, 76–79 (1990).
Cohen, A. et al. Blood 78, 94–102 (1991).
Touw, J. et al. Blood 75, 2097–2101 (1990).
Grimaldi, J. C. & Meeker, T. C. Blood 73, 2081–2085 (1989).
Dadi, H., Ke, S. & Roifman, C. M. Biochem. biophys. Res. Commun. 192, 459–464 (1993).
Burke, T. et al. J. med. Chem. 36, 425–432 (1993).
Cushman, M. et al. J. med. Chem. 37, 3353–3362 (1994).
Osherove, N. & Levitzki, A. Eur. J. Biochem. 225, 1047–1053 (1994).
Padeh, S., Levitzki, A., Gazit, A., Mills, G. & Roifman, C. M. J. clin Invest. 87, 1114–1118 (1991).
Osamu, M. et al. Blood 84, 1501–1507 (1994).
Sato, M. et al. J. exp. Med. 180, 2101–211 (1994).
Barber, D. L. & D'Andrea, A. D. Molec. cell. Biol. 14, 6506–6514 (1994).
Tanaka, N. et al. Proc. natn. Acad. Sci. U.S.A. 91, 7271–7275 (1994).
Johnston, J. A. et al. Nature 370, 151–153 (1994).
Witthuhn, B. A. et al. Nature 370, 153–157 (1994).
Roifman, C. M., Wang, G., Freedman, M. & Pan, Z. J. Immun. 148, 1136–1142 (1992).
Wilks, A. F. et al. Molec. cell. Biol. 11, 2057–2065 (1991).
Harpur, A. G. Andres, A-C., Ziemiecki, A., Aston, R. R. & Wilkes A. F. Oncogene 7, 1347–1353 (1992).
Rothman, P. et al. Immunity 1, 457–468 (1994).
Bolen, J. B., Thompson, P. A., Eiseman, E. & Horak, I. D. Adv. Cancer Res. 57, 103–149 (1991).
Bolen, J. B. et al. FASEB J. 6, 3403–3409 (1992).
Estrov, Z. & Freedman, M. H. Expl Hemat. 19, 221–225 (1991).
Schmid, I., Uittenbogaart, C. H. & Giorgi, J. V. Cytometry 15, 12–20 (1994).
Uckun, F. M. et al. Science 267, 886–891 (1995).
Kamel-Reid, S. et al. Science 246, 1597–1600 (1989).
Kamel-Reid, S. et al. Leukemia 6, 8–17 (1992).
Arpaia, E., Shahar, M., Dadi, H., Cohen, A. & Roifman, C. M. Cell 76, 947–958 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meydan, N., Grunberger, T., Dadi, H. et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379, 645–648 (1996). https://doi.org/10.1038/379645a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/379645a0
This article is cited by
-
Medicinal chemistry perspective of JAK inhibitors: synthesis, biological profile, selectivity, and structure activity relationship
Molecular Diversity (2024)
-
Angiopathic activity of LRG1 is induced by the IL-6/STAT3 pathway
Scientific Reports (2022)
-
The expression and the tumor suppressor role of CLDN6 in colon cancer
Molecular and Cellular Biochemistry (2022)
-
Cytokine storm: behind the scenes of the collateral circulation after acute myocardial infarction
Inflammation Research (2022)
-
Complement C3 overexpression activates JAK2/STAT3 pathway and correlates with gastric cancer progression
Journal of Experimental & Clinical Cancer Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.