Abstract
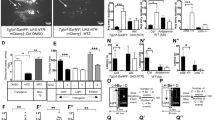
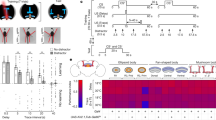
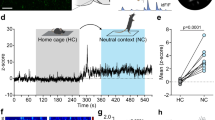
ENVIRONMENTAL light is the dominant temporal cue for the entrainment of circadian rhythms. In mammals, light entrains circadian rhythms by daily resetting a pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN)1,2. Although it is widely held that phase resetting by light involves cellular elements within the SCN that are uniquely responsive to photic cues1,3, we now report that non-photic cues that reliably precede the onset of light can, through associative learning, come to activate these elements. In rats, a neutral non-photic stimulus paired with light in pavlovian conditioning trials was capable of eliciting cellular and behavioural effects characteristic of phase-dependent resetting of the pacemaker by light, the expression of the transcription factor Fos in SCN cells, and phase shifts in free-running activity and temperature rhythms4–7. Thus an associative learning process, pavlovian conditioning, provides a means whereby environmental cues that predict light onset can come to mimic the effect of light on the SCN pacemaker and thereby bring about entrainment of circadian rhythms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Morin, L. P. Brain Res. Rev. 67, 102–127 (1994).
Pittendridge, C. S. in Handbook of Behavioral Neurobiology Biological Rhythms (ed. Ashchoff, J.) 95–124 (Plenum, New York, 1981).
Takahashi, J. S. A. Rev. Neurosci. 18, 531–553 (1995).
Komhouser, J. M., Nelson, D. E., Mayo, K. E. & Takahashi, J. S. Neuron 5, 127–134 (1990).
Rusak, B., Robertson, H. A., Wisden, W. & Hunt, S. P. Science 248, 1237–1240 (1990).
Schwartz, W. J., Takeuchi, J., Shannon, W., Davis, E. M. & Aronin, N. Neuroscience 58, 573–583 (1994).
Amir, S. & Robinson, B. Neuroscience 69, 1005–1011 (1995).
Moore, R. Y. & Lenn, N. J. J. comp. Neurol. 146, 1–14 (1972).
Johnson, R. F., Moore, R. Y. & Morin, L. P. Brain Res. 462, 301–312 (1988).
Card, J. P. & Moore, R. Y. J. comp. Neurol. 284, 135–147 (1989).
Moore, R. Y. & Card, J. P. J. comp. Neurol. 344, 403–430 (1994).
Rusak, B., Meijer, J. H. & Harrington, M. E. Brain Res. 493, 283–291 (1989).
Harrington, M. E. & Rusak, B. J. J. biol. Rhythms 1, 309–325 (1986).
Picard, G. E. Neurosci. Lett. 55, 211–217 (1985).
Hickey, T. L. & Spear, P. D. Expl Brain Res. 24, 523–529 (1976).
Shinohara, K., Tominga, K., Fukuhara, C., Otori, Y. & Inouye, S.-I. T. Neuroscience 56, 813–822 (1993).
Harrington, M. E., Nance, D. M. & Rusak, B. Brain Res. 410, 275–282 (1987).
Card, J. P. & Moore, R. Y. J. comp. Neurol. 206, 390–396 (1982).
Edelstein, K. & Amir, S. Brain Res. 600, 254–258 (1995).
Davidowa, H. & Albrecht, D. Behavl Brain Res. 50, 127–133 (1992).
Moga, M. M., Weis, R. P. & Moore, R. Y. J. comp. Neurol. 359, 221–238 (1995).
Mrosovsky, N. J. comp. Physiol. A 162, 35–46 (1988).
Wickland, C. R. & Turek, F. W. Am. J. Physiol. 261, R1109–R1117 (1991).
Van Reeth, O. & Turek, F. W. Nature 339, 49–51 (1989).
Zhang, Y., Van Reeth, O., Zee, P. C., Takahashi, J. S. & Turek, F. W. Neurosci. Lett. 164, 203–208 (1993).
Janik, D. & Mrosovsky, N. NeuroReport 3, 575–578 (1992).
Mead, S. et al. J. Neurosci. 12, 2516–2522 (1992).
Cutrera, R. A., Kalsbeek, A. & Pévet, P. Brain Res. 602, 14–20 (1993).
Daan, S. & Pittendrigh, C. S. J. comp. Physiol. 106, 253–266 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amir, S., Stewart, J. Resetting of the circadian clock by a conditioned stimulus. Nature 379, 542–545 (1996). https://doi.org/10.1038/379542a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/379542a0
This article is cited by
-
Potent social synchronization can override photic entrainment of circadian rhythms
Nature Communications (2016)
-
Reciprocal Inhibitory Interactions Between the Reward-Related Effects of Leptin and Cocaine
Neuropsychopharmacology (2016)
-
Circadian rhythms in fungi
Journal of Genetics (1996)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.