Abstract
We screened for genetic alterations of adenomatous polyposis coli (APC) and β-catenin genes in 17 frozen specimens from 12 cases of sporadic desmoid tumors and then subdivided these cases into two groups according to the results of mutational analysis. We further examined mRNA expression of β-catenin and cyclin D1 by TaqMan PCR and compared the mRNA expression within both groups. Single-strand conformation polymorphism analysis followed by DNA direct sequencing revealed β-catenin mutation in 3 of 12 cases (6 of 17 specimens), whereas no APC missense mutations in the mutation cluster region were found. TaqMan PCR revealed extremely higher mRNA expression of β-catenin and cyclin D1 in desmoid tumors, compared with those of normal skeletal muscles. In the β-catenin mutated group, cyclin D1 mRNA expression was significantly higher than that of the β-catenin wild-type group (p = 0.0120, Mann-Whitney U test). In addition, in the β-catenin mutated group, β-catenin mRNA expression was also significantly higher than that of the β-catenin wild-type group (p = 0.0036, Mann-Whitney U test). All cases of desmoid tumors showed detectable β-catenin nuclear expression immunohistochemically. These results suggest that a continuously elevated β-catenin protein level caused by the β-catenin mutation itself may have a stronger power that can transactivate transcription in vivo. Furthermore, the results provide a possible association between higher β-catenin mRNA expression and mutated β-catenin in sporadic desmoid tumors. This may suggest that the β-catenin gene may be up-regulated by mutated or continuously elevated β-catenin protein, that is, the β-catenin gene may also be one of the targeted genes in the APC-β-catenin-Tcf pathway.
Similar content being viewed by others
Introduction
Beta-catenin is a multifunctional protein involved in the wingless/Wnt signal transduction pathway, in addition to being a cell-cell adhesion regulator when binding to E-cadherin adhesion molecules (Brabletz et al, 2000; Hirohashi, 1998). The binding of β-catenin to adenomatous polyposis coli (APC) protein requires phosphorylation of β-catenin by glycogen synthase kinase (GSK)-3β on serine/threonine residues. Mutations of APC or β-catenin result in stabilization of β-catenin and a significant accumulation of this protein within the cytoplasm (Fukuchi et al, 1998; Koch et al, 1999; Miyoshi et al, 1998b; Morin et al, 1997; Palacios and Gamallo, 1998).
Desmoid tumor is an infiltrative and locally aggressive lesion characterized by a florid fibroblastic proliferation. Although desmoid tumor frequently recurs after surgical resection, it never results in distant metastasis. Desmoid tumors are also known to have a high frequency of APC and β-catenin mutations and show elevated β-catenin protein (Alman et al, 1997; Giarola et al, 1998; Miyoshi et al, 1998a; Shitoh et al, 1999; Tejpar et al, 1999), which could be thought of as providing an in vivo model system for the APC-β-catenin-Tcf pathway. Furthermore, Li et al (1998) demonstrated that APC truncating mutations provided aggressive fibromatosis cells with a proliferative advantage through β-catenin, and they suggested that β-catenin acted to transactivate transcription. Some of the targeted genes of the APC-β-catenin-Tcf pathway have been identified in vitro (Brabletz et al, 1999; Crawford et al, 1999; He et al, 1998; Tetsu and McCormick, 1999), however, it is still unclear how the accumulated β-catenin could transactivate transcription of specific genes such as cyclin D1, c-myc, and MMP-7, in vivo.
We have recently demonstrated that the β-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumors (Saito et al, 2001). Furthermore, some cases of sporadic desmoid tumors with β-catenin mutation showed cyclin D1 overexpression, although immunohistochemically showing focal β-catenin nuclear expression (Saito et al, 2001). In addition, an increased β-catenin protein level is thought to be caused by posttranscriptional factors such as APC or β-catenin mutations (Morin et al, 1997) or Wnt signaling, which mediates the inhibition of GSK-3β (Moon et al, 1997). However, the relationship between the elevated β-catenin protein and β-catenin mRNA expression has rarely been discussed (Alman et al, 1997).
In this study, we screened for genetic alterations of APC and β-catenin genes in 17 specimens from 12 cases of sporadic desmoid tumors and then subdivided these cases into two groups according to the results of mutational analysis. We further examined mRNA expressions of β-catenin and cyclin D1, which is one of the candidates for target genes of the APC-β-catenin-Tcf pathway, by TaqMan PCR, and compared the mRNA expression in both groups. The purpose of this study was to examine the relationship between elevated β-catenin protein level and mRNA expression of a specific target gene, cyclin D1, in vivo, and also β-catenin mRNA expression.
Results
β-Catenin and APC Gene Mutations
The results are summarized in Table 1. Single-strand conformation polymorphism (SSCP) analysis followed by DNA direct sequencing revealed β-catenin mutations in 6 of 17 samples (in 3 of 12 patients) (Fig. 1). All four samples obtained from one patient (Case 1), who had suffered local recurrence three times, contained β-catenin mutation at codon 45 (TCT to TTT). In addition, β-catenin mutations at codon 42 (ACA to AGA) or at codon 45 (TCT to TTT) were observed in one case each. However, no interstitial deletion of β-catenin gene was detected. On the other hand, APC missense mutations in the mutation cluster region (MCR) of the APC gene were not found, although polymorphisms at codon 1493 (ACG to ACA, Thr to Thr) were observed in 10 of 17 samples (in 7 of 12 patients). None of the three control samples from normal skeletal muscles contained any β-catenin or APC missense mutations.
Sequencing results for exon 3 of β-catenin in desmoid tumors. Tumor sequences showing the substitution of AGA for ACA (arrow, lower left) at codon 42 in Case 12. Tumor sequence showing the substitution of TTT for TCT (arrow, lower right) at codon 45 in Case 1. Corresponding normal sequences are also shown (arrows, upper section).
Semiquantitative Assay for β-Catenin and Cyclin D1 mRNA
The results from semiquantitative assays are also summarized in Table 1. The cases were divided into two groups according to the results of mutational analysis: β-catenin mutated group and β-catenin wild-type group. Figure 2A shows the scattergram of cyclin D1 mRNA expression in the β-catenin mutated group, the β-catenin wild-type group, and normal skeletal muscles. In the β-catenin mutated group, cyclin D1 mRNA expression was significantly higher than that of the β-catenin wild-type group (p = 0.0120). In addition, Figure 2 B shows the scattergram of β-catenin mRNA expression in the β-catenin mutated group, the β-catenin wild-type group, and normal skeletal muscles. In the β-catenin mutated group, β-catenin mRNA expression was also significantly higher than that of the β-catenin wild-type group (p = 0.0036). Furthermore, the effect of APC codon 1493 polymorphism upon mRNA expression of β-catenin or cyclin D1 was also evaluated, however, this polymorphism did not affect the mRNA expression (data not shown). In addition, the mRNA expression of β-catenin and cyclin D1 in normal skeletal muscles was extremely lower, compared with that of desmoid tumors.
A and B, Scattergram of cyclin D1 (A) and β-catenin (B) mRNA expression in sporadic desmoid tumors according to the status of genetic alteration of the β-catenin gene. The value of cyclin D1 or β-catenin mRNA expression was standardized to that of GAPDH mRNA expression. Cyclin D1 and β-catenin mRNA levels in the β-catenin mutated group were significantly higher than those of the β-catenin wild-type group (p = 0.0120, p = 0.0036, respectively, Mann-Whitney U test). (+) β-catenin mutated group; (-) β-catenin wild-type group; M, normal skeletal muscles.
Western Blot Analysis for β-Catenin
To investigate whether β-catenin protein levels are actually increased in these samples with β-catenin mutation, we also performed Western blot analysis in all samples of desmoid tumor and normal skeletal muscles. Western blot analysis showed increased levels of β-catenin protein in six samples with β-catenin mutation compared with the samples without β-catenin mutation or normal skeletal muscles (Fig. 3).
Immunohistochemistry
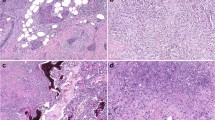
Immunohistochemisty showed β-catenin nuclear staining in the fibroblastic cells of all cases of desmoid tumors (Fig. 4, A and B). There was little difference in β-catenin nuclear staining between the primary and recurrent cases from the same patients. Some sections from the cases without β-catenin mutation showed β-catenin nuclear expression more diffusely than the cases with β-catenin mutation. The percentage of positively stained tumor cells in each case is described in Table 1.
A and B, Immunohistochemical staining of β-catenin in sporadic desmoid tumors. Almost all of the tumor cells (approximately 90%) throughout the lesion are positively stained for β-catenin in Case 1 (primary lesion) with β-catenin mutation at codon 45 (A). Most of the tumor cells (approximately 80%) are also positively stained for β-catenin in Case 6 with wild-type β-catenin (B).
Discussion
The stabilized form of β-catenin that cannot be degraded by APC transactivates transcription of the members of the TCF-LEF family, which are members of the high mobility group, in desmoid tumors (Li et al, 1998). Cyclin D1 is one of the candidates for the targeted genes of the APC-β-catenin-Tcf pathway (Tetsu and McCormick, 1999). We have recently demonstrated that β-catenin nuclear accumulation or β-catenin mutations are necessary for cyclin D1 overexpression in sporadic desmoid tumors, providing the possibility that cyclin D1 is one of the targeted genes of the APC-β-catenin-Tcf pathway in desmoid tumors (Saito et al, 2001). In this study, cyclin D1 mRNA expression in desmoid tumors was much higher than that in normal skeletal muscles, suggesting that accumulated β-catenin, regardless of the mutational status in β-catenin and APC genes, can also transactivate transcription. Furthermore, cyclin D1 mRNA levels in cases with mutated β-catenin were significantly higher than those with wild-type β-catenin. All cases of sporadic desmoid tumors showed diffuse β-catenin nuclear staining immunohistochemically, regardless of mutation status. Moreover, some of the cases with wild-type β-catenin showed more diffuse β-catenin accumulation than the cases with mutated β-catenin. Altogether, these results suggest that mutated β-catenin has some advantage regarding transactivating transcription over wild-type β-catenin in sporadic desmoid tumors.
The most striking finding in this study is that β-catenin mRNA levels in cases with mutated β-catenin were also significantly higher than those with wild-type β-catenin, with a stronger statistical power than that of cyclin D1. The increased protein level of β-catenin is thought to have been caused by APC mutations, by β-catenin mutations themselves (Morin et al, 1997), by Wnt signal activation (Moon et al, 1997), or by decreased mRNA degradation. These are all posttranscriptional factors. The relationship between increased β-catenin protein level and its transcriptional level has rarely been discussed. Alman et al (1997) examined APC mutations, β-catenin protein levels, and mRNA levels in six cases of aggressive fibromatosis without familial adenomatous polyposis (FAP) and reported that all six cases had a higher level of β-catenin protein than the surrounding normal tissues, despite containing similar levels of β-catenin mRNA, although they did not examine β-catenin mutations in these cases. The β-catenin protein levels in half of their cases were thought to have been elevated by APC mutations, however, it is unclear that β-catenin protein in their cases was really continuously elevated and resistant to degradation. In this study, we have for the first time described, semiquantitatively by TaqMan PCR, a possible association between β-catenin mutation status and its mRNA expression level.
Recently, the function of APC protein has been known to be compensated by axin, the other component that has tumor suppressor function in the β-catenin/GSK-3β/APC/axin complex (Hart et al, 1998; Nakamura et al, 1998; Satoh et al, 2000). On the other hand, β-catenin with mutant Ser/Thr phosphorylation sites in exon 3 has been reported to be resistant to degradation (Korinek et al, 1997; Morin et al, 1997; Satoh et al, 2000). In addition, β-catenin mutations occurring at the neighboring sites of the Ser/Thr residues, as was observed in our study, have also been reported to be resistant to degradation (Fukuchi et al, 1998; Iwao et al, 1998; Miyoshi et al, 1998b; Palacios and Gamallo, 1998). Therefore, β-catenin protein levels in our cases with β-catenin mutations can be considered to be continuously increased, compared with the cases of Alman et al (1997). Indeed in this study, we could confirm by Western blot analysis that β-catenin protein levels in samples with mutated β-catenin have increased compared with the samples with wild-type β-catenin. The increased β-catenin mRNA levels in cases with mutant β-catenin may suggest that β-catenin transcription is up-regulated by continuously elevated β-catenin. Alternatively, the mutated β-catenin may be specifically involved in transactivating transcription of the β-catenin gene, although evidence concerning the speed of β-catenin mRNA degradation was not provided by our study. However, further studies are necessary to show whether continuously elevated β-catenin can transactivate transcription of the β-catenin gene itself.
β-catenin and cyclin D1 mRNA expression levels in the recurrent three lesions with wild-type β-catenin that were obtained from one patient (Case 2) who had suffered local recurrence four times were almost at the same level. In contrast, in four lesions containing β-catenin mutations that were obtained from another patient (Case 1) who had suffered local recurrence three times, the values of β-catenin and cyclin D1 mRNA expression fell within a fairly wide range. The mRNA expression of β-catenin and cyclin D1 in normal skeletal muscles with wild-type β-catenin and APC genes was much lower in this study. Therefore, these differences in samples from the same patient may be considered the result of contamination of the surrounding normal tissue as a result of the surgical treatment, because desmoid tumor is a monoclonal proliferation of fibroblastic cells.
The β-catenin mutation rate in this study was 25% (3/12). This rate is lower than those of the previously reported values (approximately 50%: Miyoshi et al, 1998a; Tejpar et al, 1999). This difference is likely caused by a sampling error from examining a relatively small frozen sample number. In fact, in our initial report of β-catenin mutation in sporadic desmoid tumor, which was based on formalin-fixed, paraffin-embedded materials and included samples used in this study, the β-catenin mutation rate was 38.9% (7/18) (Saito et al, 2001).
In conclusion, we examined the genetic alterations in exon 3 of the β-catenin gene and in the MCR of APC gene in sporadic desmoid tumors and compared mRNA expression of β-catenin and cyclin D1 according to the presence of genetic alterations of these genes. This study provides for the first time a possible association between higher β-catenin mRNA expression and mutated β-catenin, in sporadic desmoid tumors, which could be an in vivo model system for the APC-β-catenin-Tcf pathway. The β-catenin gene may also be one of the targeted genes in the APC-β-catenin-Tcf pathway, although it is unclear whether it is a direct or indirect targeted gene.
Materials and Methods
Materials
Seventeen specimens from 12 patients with sporadic extra-abdominal or abdominal wall desmoid tumors without FAP, for which frozen materials were available, were selected for relative quantitative real-time PCR assay from among the collection of soft-tissue tumors registered in the Department of Anatomic Pathology, Pathological Sciences, Graduate School of Medical Sciences, Kyushu University, Japan. Fresh samples were carefully dissected from the tumors so as not to include the surrounding normal tissue, and these were frozen in liquid nitrogen immediately and stored at −80° C. Fresh-frozen materials from three cases of normal skeletal muscle tissue were used for the control. Diagnosis of all the cases was based on light microscopic examinations with hematoxylin-eosin staining.
PCR-SSCP and Mutational Analysis of the APC Gene and β-Catenin Genes
Genomic DNA was purified from 17 specimens of frozen material from desmoid tumors using standard proteinase K digestion and phenol/chloroform extraction after homogenization. PCR-SSCP was performed for the MCR of the APC gene exon 15 and for the entire region of the β-catenin gene exon 3 using a previously described pair of primers (Iwao et al, 1998; Yagi et al, 1997). PCR was carried out for 40 cycles after an initial denaturing at 96° C for 5 minutes. Each cycle consisted of denaturation at 96° C for 1 minute, annealing at 55° C (APC gene) or 58° C (β-catenin gene) for 1 minute, and extension at 72° C for 1 minute. After the final cycle of amplification, the extension was continued for an additional 7 minutes at 72° C. Human genomic DNA (CLONTECH, Palo Alto, California) was used as a positive control for each PCR and for the subsequent reactions. We also confirmed that there was no contamination in any PCR or the subsequent reactions by using distilled water instead of the template DNA. SSCP was performed using a DNA fragment analyzer (GenePhor, Amersham Pharmacia Biotech, Uppsala, Sweden) at 600 V, 25 mA, 15W and 5° C, for 120 minutes, and then visualized by a DNA Silver Staining Kit (GenePhor, Amersham Pharmacia Biotech) (Saito et al, 2000). To increase the quantity of mutant DNA before sequencing, extra bands that seemed to be aberrantly migrating were excised from the SSCP gel and reamplified for 25 cycles under the same conditions. The samples were analyzed for sequencing after the subsequent reaction. The sequence data were collected by ABI Prism 310 Collection Software and were analyzed by Sequencing Analysis and Sequence Navigator Software (Perkin Elmer, Norwalk, Connecticut).
RNA Extraction and Reverse Transcription
Total RNA was extracted from 17 samples of sporadic desmoid tumors and 3 cases of human normal skeletal muscle, using Trizol Reagent (GIBCO BRL, Tokyo, Japan) according to the manufacturer’ s protocol. Five micrograms of RNA of each sample were used for the subsequent reverse transcription. After the reaction, RNase treatment was performed to eliminate RNA.
TaqMan PCR to Detect mRNA Quantity of β-Catenin and Cyclin D1
Semiquantitative PCR for β-catenin and cyclin D1 was performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, California) and predeveloped TaqMan assay reagents (β-catenin: human beta-catenin; cyclin D1: human cyclin D1; and GAPDH: human GAPDH). The PCR reaction was carried out according to the manufacturer’s protocol. The standard curve was constructed with serial dilutions of one of the cDNA samples of human normal skeletal muscle. The obtained data were standardized by using data of the internal housekeeping gene, GAPDH. The final numerical value (V) in each sample was calculated as follows: V = β-catenin or cyclin D1 mRNA value/GAPDH mRNA value.
Western Analysis for β-Catenin
Frozen materials were solubilized in lysis buffer (20 mM Tris [pH 7.4], 250 mM NaCl, 1.0% NP40, 1 mM EDTA, 50 mg/ml leupeptin, 1 mM PMSF, 1 mM sodium orthovanadate, and 1 mM NaF) and then incubated on ice for 10 minutes. After homogenization, materials were clarified by centrifugation at 14000 rpm for 10 minutes at 4° C. Protein quantity was determined using the Bradford protein assay kit (Bio-Rad, Hercules, California). The samples were heated at 85° C for 5 minutes, and 50 μg of total protein from each sample was run on a 4% to 12% gradient 3-(N-morpholino) propane sulfonic acid (MOPS)-polyacrylamide gel (Novex, San Diego, California) and blotted onto nitrocellulose filters (Amersham, Arlington Heights, Illinois). The filters were pretreated with Tris-buffered saline containing 5% dry milk and 0.05% Triton-X for 1 hour at room temperature, then incubated with anti-β-catenin mouse monoclonal antibody (clone 14, 1:200; Transduction Laboratories, Lexington, Kentucky) for 1 hour at room temperature. After being washed several times, the filters were incubated with the horseradish peroxidase-conjugated secondary antibody (Biosource, Camarillo, California). After the final washing, the immunoreactivity of the blots was detected using an enhanced chemiluminescence system (Amersham).
Immunohistochemistry
Immunohistochemistry was performed for formalin-fixed, paraffin-embedded material of each case, using anti-β-catenin mouse monoclonal antibody. For evaluating β-catenin staining, each section was semiquantitatively scored to the nearest 10%, according to the percentage of positively stained tumor cells showing nuclear expression.
Statistical Analysis
The correlations between each group and mRNA expression were determined by using the Mann-Whitney U test. Probability values of less than 0.05 were considered as significant.
References
Alman BA, Li C, Pajerski ME, Diaz-Cano S, and Wolfe HJ (1997). Increased β-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumor). Am J Pathol 151: 329–334.
Brabletz T, Herrmann K, Jung A, Faller G, and Kirchner T (2000). Expression of nuclear β-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol 156: 865–870.
Brabletz T, Jung A, Dag S, Hlubek F, and Kirchner T (1999). β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155: 1033–1038.
Crawford HC, Fingleton BM, Rudolph-Owen LA, Heppner Goss KJ, Rubinfeld B, Polakis P, and Matrisian LM (1999). The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumor. Oncogene 18: 2883–2891.
Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, and Hirohashi S (1998). β-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 58: 3526–3528.
Giarola M, Wells D, Mondini P, Pilotti S, Sala P, Azzarelli A, Bertario L, Pierotti MA, Delhanty JDA, and Radice P (1998). Mutations of adenomatous polyposis coli (APC) gene are uncommon in sporadic desmoid tumors. Br J Cancer 78 (5): 582–587.
Hart MJ, de los Santos R, Albert IN, Rubinfeld B, and Polakis P (1998). Downregulation of β-catenin by human axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol 8: 573–581.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, and Kinzler KW (1998). Identification of c-myc as a target of the APC pathway. Science 281: 1509–1512.
Hirohashi S (1998). Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 153: 333–339.
Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, and Miyoshi Y (1998). Activation of the β-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res 58: 1021–1026.
Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, and Pietsch T (1999). Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the β-catenin gene. Cancer Res 59: 269–273.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, and Clevers H (1997). Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787.
Li C, Bapat B, and Alman BA (1998). Adenomatous polyposis coli gene mutation alters proliferation through its beta-catenin-regulatory function in aggressive fibromatosis (desmoid tumor). Am J Pathol 153: 709–714.
Miyoshi K, Iwao K, Nawa G, Yoshikawa H, Ochi T, and Nakamura Y (1998a). Frequent mutation in the beta-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res 10: 591–594.
Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, and Nakamura Y (1998b). Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 58: 2524–2527.
Moon RT, Brown JD, and Torres M (1997). WNTs modulate cell fate and behavior during vertebrate development. Trends Genet 13: 157–162.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, and Kinzler KW (1997). Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275: 1787–1790.
Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, and Akiyama T (1998). Axin, an inhibitor of the Wnt signaling pathway, interacts with β-catenin, GSK-3β and APC and reduces the β-catenin level. Genes Cells 3: 395–403.
Palacios J and Gamallo C (1998). Mutation in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 58: 1344–1347.
Saito T, Oda Y, Sakamoto A, Tamiya S, Kinukawa N, Hayashi K, Iwamoto Y, and Tsuneyoshi M (2000). Prognostic value of the preserved expression of the E-cadherin and catenin families of adhesion molecules and of β-catenin mutations in synovial sarcoma. J Pathol (192):342–350.
Saito T, Oda Y, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, and Tsuneyoshi M (2001). β-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumors. J Pathol (195):222–228.
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, and Nakamura Y (2000). AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 24: 245–250.
Shitoh K, Konishi F, Iijima T, Ohdaira T, Sakai K, Kanazawa K, and Miyaki M (1999). A novel case of a sporadic desmoid tumor with mutation of the beta catenin gene. J Clin Pathol 52: 695–696.
Tejpar S, Nollet F, Li C, Wunder JS, Michils G, Cin PD, Cutsem EV, Bapat B, Roy FV, Cassiman JJ, and Alman BA (1999). Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 18: 6615–6620.
Tetsu O and McCormick F (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426.
Yagi OK, Akiyama Y, Ohkura Y, Ban S, Endo M, Saitoh K, and Yuasa Y (1997). Analyses of the APC and TGF-β type II receptor genes, and microsatellite instability in mucosal colorectal carcinoma. Jpn J Cancer Res 88: 718–724.
Acknowledgements
We are grateful to Miss M. Okano for her excellent technical assistance. We thank Miss Katherine Miller, Royal English Language Centre, Fukuoka, Japan, for revising the English used in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by a Grant-in-Aid for Cancer Research from the Fukuoka Cancer Society and a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (12670167).
Rights and permissions
About this article
Cite this article
Saito, T., Oda, Y., Kawaguchi, Ki. et al. Possible Association Between Higher β-Catenin mRNA Expression and Mutated β-Catenin in Sporadic Desmoid Tumors: Real-Time Semiquantitative Assay by TaqMan Polymerase Chain Reaction. Lab Invest 82, 97–103 (2002). https://doi.org/10.1038/labinvest.3780399
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780399
This article is cited by
-
Molecular Pathogenesis of Sporadic Desmoid Tumours and Its Implications for Novel Therapies: A Systematised Narrative Review
Targeted Oncology (2022)
-
Papillary thyroid carcinoma with nodular fasciitis-like stroma and β-catenin mutations should be renamed papillary thyroid carcinoma with desmoid-type fibromatosis
Modern Pathology (2017)
-
Desmoplastic fibroma of the rib with cystic change: a case report and literature review
Skeletal Radiology (2014)
-
Fibromatoses desmoïdes: étude immunohistochimique de 14 cas sur tissue microarray (TMA)
Bio tribune magazine (2010)
-
FAP-associated desmoid invasiveness correlates with in vitro resistance to doxorubicin
Familial Cancer (2009)