Abstract
The backbone of daily pathological diagnostic work is the paraffin section. Paraffin sections are still prepared by methods largely unchanged for over 150 years. A xylene-free method has been developed that excludes xylene, not only as the intermediate step before the paraffin baths, but also for deparaffinizing of the cut sections, which also eliminates the need for rehydration and dehydration for the staining and mounting steps. Elimination of xylene from tissue processing cuts costs, saves time, and improves the laboratory environment. Experience with xylene-free sections since 1995 at the Vrinnevi Hospital is favorable. Our opinion is that the xylene-free sections are equivalent to conventionally processed sections. To test this hypothesis, nine pathologists from three hospitals participated in an evaluation trial. Paired tissue blocks from 10 consecutively submitted samples each of breast, intestine, and skin were processed by either the xylene-free or the conventional method. Sections from each block were deparaffinized and stained with hematoxylin-eosin (H&E), with periodic acid-Schiff (PAS), and with van Gieson’s method. A randomized mix of 180 sections (10 samples × 3 tissues × 3 stains × 2) gave 90 matched pairs. Each section was blindly examined and scored by nine pathologists to give 810 paired observations for statistical evaluation. The xylene-free sections were ranked as good as or better than their conventional counterparts in 74% of the comparisons, and poorer in 26%. The major discriminating factor was the staining method. H&E and PAS sections were equivalent. The xylene-free van Gieson sections, cut from the same blocks and randomly assigned to this stain, tended to be downgraded. This could be traced to a faulty stain solution used for this batch. The overall results have demonstrated professional acceptance for the xylene-free method of processing histological sections.
Similar content being viewed by others
Introduction
During the previous few decades, ever more sophisticated, and costly, immunological and molecular biological techniques have been introduced into pathological practice and, when appropriately applied, have given precision in diagnosis and refinements in patient care. The basic material for the bulk of daily diagnostic work for most pathologists, however, remains the paraffin section, usually stained with hematoxylin-eosin (H&E). Methods of preparing these sections have remained largely unchanged for 150 years.
The xylene-free method for paraffin sections, developed and in use at Vrinnevi Hospital since 1995 (Falkeholm, 1996), addresses underlying problems common to all laboratories nowadays: cost containment, turnaround time, and working environment. Our working hypothesis is that xylene-free histological sections are on a par with conventional sections. The advantage is not in the sections themselves but in the savings to be gained.
To test this hypothesis, parallel xylene-free and conventional processing, from embedding and deparaffinization to staining and mounting, was applied to identical tissue samples. The final slides, in random order, were then evaluated blindly by a panel of pathologists from three different hospitals. The xylene-free sections were deemed to be an acceptable alternative to the conventional.
Results
Each of nine pathologists examined 180 histological sections (90 for each method) to give a total of 1620 assessments. These represent 810 paired observations for statistical evaluation as described in “Materials and Methods.”
Wilcoxon Signed Ranks Test
The xylene-free sections were judged to be as good as or better than the matching conventional sections in 74% of the comparisons (462 + 136), and poorer in 26% (Table 1).
Comparison of Numerical Scores
Because the middle score of 2 was intended to represent the “normal,” with 1 representing better and 3 poorer, both series of sections were considered on the whole to be somewhat better than average, with mean scores of 1.9 for the xylene-free and 1.8 for the conventional sections. The difference between the mean scores is numerically small (0.1) but, with the large number of comparisons, to the advantage of the conventional sections (p = 0.001).
Kappa-Statistic Measure of Agreement
The Cohen’s kappa is often used for measuring agreement better than chance (Altman, 1991). Kappa has a maximum of one when agreement is perfect, whereas a value of zero indicates no agreement better than chance. We give kappa a 95% confidence interval. A problem with kappa is that the value depends upon the prevalences (Altman, 1991). Because of this, we also give the absolute agreement as a measure, which is the number of agreed comparisons divided by number of total comparisons.
A substantial core of agreement emerged when both members of a matched pair of slides were scored as acceptable or more than acceptable. From a practical point of view, these assessments can be lumped together to highlight the asymmetric situation where one member of a pair was scored as acceptable or more than acceptable, whereas the other was downgraded to less than acceptable. See Figure 1 as a guide to the interpretation of the following tables.
Guide to the interpretation of the kappa tables when looking at agreement between methods. The absolute numbers of acceptable or downgraded xylene-free and conventional histological sections can be compared with each other or considered in relation to the totals. The kappa coefficient can be considered in relation to the degree of observed agreement (ie, ties for “acceptable” plus ties for “less than acceptable” divided by the total number of paired observations) and to the 95% confidence interval (CI95 = kappa ± 1.96 × se).
Table 2 applies this approach for an overall comparison of the results for the whole material and by all pathologists. There is considerable agreement for acceptable or more than acceptable, but much less agreement for less than acceptable. Of the 197 nontied pairs, where one of the pair was classed as less than acceptable, the xylene-free section received a better score in 72 comparisons and the conventional section in 125 comparisons.
Further refinement of kappa stratifications can be applied for analysis of this divergence. Are there systematic factors involving preparation method or particular stains or tissues, or are there observer or institutional preferences?
Of all the parameters possibly influencing the differences in scoring, staining method, in particular van Gieson’s, emerged as the most discriminating for the whole material and all pathologists (Table 3), and on an institutional basis (Table 4).
For the whole material (Table 3), there is a more even distribution for the paired xylene-free and conventional sections stained with H&E and PAS than for those stained by van Gieson’s method. For the van Gieson sections, there is a much wider splitting of the scores when one of the pair was downgraded to the score less than acceptable: 62 xylene-free versus 10 conventional.
On institutional basis, for the six pathologists from one hospital and the remaining three from the other two hospitals (Table 4), there is the same pattern of dissatisfaction with the xylene-free van Gieson sections when one of a van Gieson pair was downgraded to less than acceptable. A lesser degree of institutional preference is evident for the H&E and PAS stains. The six pathologists from the Jönköping hospital were actually comparing the xylene-free sections with their “own” conventional sections and stains, ie, conventional sections embedded and stained in their own laboratory by their own methods. Their preference for their local variant of the H&E stain was expected a priori (see “Materials and Methods”). The other three pathologists as a group actually preferred the xylene-free H&E and PAS sections.
Individual preferences of the participating pathologists could be seen from the cross-tabulations, with a tendency for some to veer toward a generally favorable or critical score and the rest clustering toward the “normal.” Stratifying and cross-tabulating, as above, for tissue type (breast, skin, intestine) and consecutive sample number (1 to 10 for each tissue), in the same manner as above, again singled out xylene-free van Gieson sections, particularly for collagen-rich breast and skin, as accounting for most of the downgrading. The full tables are available upon request.
Discussion
The design of this trial for comparison of xylene-free and conventional histological preparation methods was intended to reflect everyday laboratory conditions. To this end, randomized histological sections from matched pairs of tissue samples were scored by a panel of pathologists. Each pathologist scored each slide as being either acceptable for diagnostic purposes (“normal”) or of better or poorer quality. The slides were presented in boxes in numerical order, ie, of randomized origin, and the evaluation of each slide was recorded for each pathologist. These subjective grades could be expected to be assigned somewhat differently by the participating pathologists, but individual preferences proved to be fairly consistent.
With this scoring system, the xylene-free sections were ranked as good as or better than the matching conventional sections in 74% of the matches and poorer in 26% (Table 1). The mean numerical scores less than 2 for both types of sections reflect general acceptance, but with a degree of preference for the conventional sections.
To analyze this pattern, the scores were stratified and cross-tabulated for kappa analysis according to the preparation method, tissue type, tissue sample, stain, and individual pathologist and institution. The results indicate considerable disparity. In only a few instances did the kappa values rise to moderate agreement; most were in the slight to fair range. The reason for this is the situation in which one of a matched pair was considered acceptable or more than acceptable, whereas the other score was less than acceptable.
The staining method could be pinpointed as the discriminating parameter. Downgrading of the xylene-free van Gieson sections was common and was dominating for tissue type and pathologist. No significant difference between the methods was evident for the H&E or PAS sections.
When this became evident during the statistical workup, one of us not previously involved in the actual scoring (CAG) blindly scored all the van Gieson sections with the same result, ie, downgrading of the xylene-free sections. The reason for this was poor discrimination between cytoplasm and collagen, but fully acceptable nuclear staining. This pinpoints the picric-acid-fuchsin component as the culprit, a well-known weakness of the van Gieson stain and one which from time to time plagues the Norrköping laboratory.
The xylene-free sections were stained on a Saturday, which was a deviation from the original plan of the trial to reflect everyday conditions, but was made necessary by the heavy routine workload at the time. Presumably, solutions made up earlier in the week were used. The conventional van Gieson sections were stained on an ordinary working day at the other participating laboratory. We accept a purely staining aberration as a reasonable explanation for the downgrading of the xylene-free van Gieson sections. The H&E and PAS sections made from the same blocks and deparaffinized in the same ways were fully comparable for the xylene-free and conventional methods. In view of the overall acceptance of the xylene-free method, we deemed it unnecessary to repeat the whole trial.
The rationale behind the xylene-free method is to produce histological sections equivalent to the conventional ones, but with the advantage of greater economical and environmental gains. Cost reduction can be attained by using and redistilling cheaper isopropanol instead of ethanol for dehydration and by eliminating expensive xylene with its attendant destruction costs. Time is saved by deparaffinizing with soap and water and using water-soluble stains. Eliminating xylene contributes toward a better working environment and, by simplifying laboratory ventilation requirements, permits greater flexibility in laboratory design and location.
In concrete terms, the saving in time for deparaffinization and staining during the early morning rush is nearly an hour. A minimum of 28 minutes was required for the xylene-free method, whereas a minimum of 80 minutes was needed for the corresponding conventional steps. The immediate saving in money can be calculated from local costs for isopropanol versus ethanol and for xylene and its disposal. Reduced needs and costs for ventilation and greater flexibility emerge when designing and building new laboratory facilities or re-locating within an existing building.
We believe that this trial demonstrates that xylene-free histological sections are qualitatively on a par with conventional paraffin sections for routine diagnostic work. In view of the savings in time, money, and the working environment, the xylene-free method meets today’s needs.
Materials and Methods
Material
Tissue blocks were taken from 10 consecutive samples, as far as possible (January 1999), of each of resected breast, intestine, and skin sample submitted for histopathological examination at the Norrköping laboratory. The only exclusion criterion was a lack of sufficient tissue, usually skin, to permit taking a tissue block thick enough to be divided into two back-to-back blocks, each 3 to 5 mm thick. Each block of the pair was placed in a separate plastic cassette and kept in formalin until processing.
Methods
Tissue Processing, Conventionally and by the Xylene-Free Method
One block of each pair was processed conventionally with xylene along with the daily routine material at the Department of Pathology, Ryhovs Hospital in Jönköping. The other block of the pair was processed together with other diagnostic material by the xylene-free method at the Norrköping laboratory. The processing methods were those in use at the respective laboratories at the time of the trial. All the paraffin blocks were then sectioned by one person in a working day using one microtome.
The embedding process is described, together with a time axis, in Figure 2. The automatic tissue processors were those in routine use at the two laboratories: a Shandon Hypercentre (Shandon Scientific, Cheshire, United Kingdom) for the conventional method in Jönköping and a Tissue-Tek VIP (Tissue-Tek, Torrance, California) for the xylene-free method in Norrköping.
The basic difference is the use of isopropanol for dehydration in the xylene-free process (puriss grade; Histolab Products, Gothenberg, Sweden) instead of increasing concentrations of ethanol followed by xylene in the conventional process. A slightly different paraffin grade from the same supplier was used at the two laboratories (Histowax: melting point 52–54° C for the xylene-free blocks, 56–58° C for the conventional, Histolab Products).
Deparaffinization, a key point in the xylene-free process, is described along a time axis for both methods in Figure 3. A large but simple waterbath with racks facilitated rapid immersion of the of the xylene-free slides.
Staining procedures for the xylene-free and the conventional sections (H&E, PAS, van Gieson’s) are outlined in Figures 4, 5 and 6. Staining of the conventional sections was preceded by rehydration and followed by dehydration before mounting. These steps are unnecessary for the xylene-free sections. Staining follows immediately after deparaffinization. Drying the xylene-free sections before mounting is sufficient. Because, by experience, the greatest difference between different laboratories is in the intensity and nuances of the H&E stain, an effort was made to attain a hue for the xylene-free sections stained in aqueous solutions similar to that of the conventional sections (Fig. 7).
Hematoxylin-eosin (H&E) staining procedures for xylene-free and conventionally processed histological sections. Htx, hematoxylin; AFIP, Armed Forces Institute of Pathology, 1992.
Periodic acid-Schiff (PAS) staining procedure for xylene-free and conventionally processed histological sections. AFIP, Armed Forces Institute of Pathology, 1992.
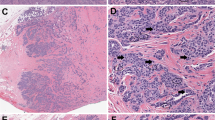
Comparison of stained xylene-free and conventional histological sections: hematoxylin-eosin (A and B), van Gieson (C and D), and PAS (E and F). These sections, taken from adjacent blocks of breast tissue (Sample 6), are the originals used in the trial. Xylene-free sections to the left, conventional to the right.
Randomization
Four cut sections from each block were each given a unique number generated randomly and then randomly assigned to the one of the three staining procedures. One slide was kept as a reserve. By this method, when the stained slides were boxed in numerical order and presented to the pathologist panel for evaluation, they formed a randomized mix of the original tissue samples, xylene-free and xylene-processed sections, and of the staining methods.
Evaluation
Nine pathologists participated: six pathologists at Ryhov Hospital, Jönköping, two at the County Hospital in Kalmar, and one not otherwise involved in this trial at Vrinnevi Hospital, Norrköping. Their professional experience ranged from 3 to 35 years, mean 19 years.
The scoring was on an arbitrary scale of 1 to 3 with the middle score “2” representing the usual, diagnostically acceptable, or “normal,” score “1” representing a better, more aesthetically pleasing level, and score “3” representing a poorer or diagnostically unacceptable level. The slides were presented in boxes in numerical order, ie, of randomized origin, and the evaluation of each slide was recorded for each pathologist on sheets given an arbitrary number not in alphabetical order.
Statistical Analysis
Each of three tissues was represented by 10 samples, each sample divided into 2. Each of these samples was stained by three methods to give a total of 180 sections, each evaluated by nine pathologists to give a total of 1620 evaluations, or 810 pairs for comparison. The scoring scale was used for a Wilcoxon signed rank test for comparison of the xylene-free sections with the conventional sections. Cohen’s kappa measure of agreement was used from the software application SPSS for Windows, release 9.0 (SPSS Inc., Chicago, Illinois).
Note Added in Proof
The manufacturer of the detergent we used for deparaffinizing has changed the formula of its product just as our article is going to press (see Fig. 3). We are concerned that the new product, which retains the trademark “Yes” or “Fairy,” does not produce the same results when used as one of the reagents in the xylene-free method reported in our paper. We have found a satisfactory alternative detergent “Ocean Handdisk,” manufactured by Kemibolaget i Farsta AB (http://www.kemibolaget.se; e-mail: info@kemibolaget.se). We have tested this product at a concentration of 20ml/l water at 90°C with good results.
We suggest that interested histopathology laboratories search their own markets for a similar product or contact Kemibolaget. At present, “Ocean Handdisk” is available in bulk from the factory but is sold to consumers in Sweden through the farmers’ cooperative Odal.
References
Altman DG (1991). Practical Statistics for Medical Research. Chapman and Hall, London: 403–409.
Armed Forces Institute of Pathology (1992). Laboratory Methods in Histotechnology. Washington, DC: American Registry of Pathology.
Falkeholm L (1996). Going green: Using water, not xylene (Letter). Lab Med 27: 638.
Acknowledgements
This work was supported by funds made available by Prof. Elvar Theodorsson, Director of Laboratory Services for Östragötland (LMÖ), Sweden.
We thank Dr. Alexei Terman, Pathology II, Medical Faculty, Linköping University, for his patience in preparing the color microphotographs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Completed with the assistance of a panel of pathologists from Ryhov Hospital, Jönköping (Penka Andreeva, Danuta Breborowicz, Ingvar Jarlfelt, Eva Nile’n, Frederik Pettersson, Hassam Pourasan), the County Hospital, Kalmar (Robert Cameron, Lennart Mellblom), and Vrinnevi Hospital, Norrköping (Jan Wågermark).
Rights and permissions
About this article
Cite this article
Falkeholm, L., Grant, C., Magnusson, A. et al. Xylene-Free Method for Histological Preparation: A Multicentre Evaluation. Lab Invest 81, 1213–1221 (2001). https://doi.org/10.1038/labinvest.3780335
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780335
This article is cited by
-
A robust nonlinear tissue-component discrimination method for computational pathology
Laboratory Investigation (2016)
-
A streptozotocin-induced diabetic neuropathic pain model for static or dynamic mechanical allodynia and vulvodynia: validation using topical and systemic gabapentin
Naunyn-Schmiedeberg's Archives of Pharmacology (2015)
-
Synthetically modified bioisosteres of salicyl alcohol and their gastroulcerogenic assessment versus aspirin: biochemical and histological correlates
Naunyn-Schmiedeberg's Archives of Pharmacology (2014)
-
Comparative evaluation of gastroulcerogenic potential of nitrogen isoforms of salicyl alcohol and aspirin in rats: biochemical and histological study
Archives of Pharmacal Research (2014)
-
Delta-24-RGD in Combination With RAD001 Induces Enhanced Anti-glioma Effect via Autophagic Cell Death
Molecular Therapy (2008)