Abstract
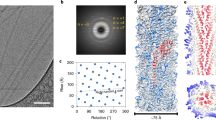
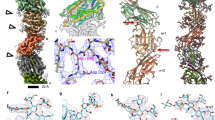
The crystallographic structure of Neisseria gonorrhoeae pilin, which assembles into the multi-functional pilus adhesion and virulence factor, reveals an α–β roll fold with a striking 85 Å α-helical spine and an 0-linked disaccharide. Key residues stabilize interactions that allow sequence hypervariability, responsible for pilin's celebrated antigenic variation, within disulphide region β-strands and connections. Pilin surface shape, hydrophobicity and sequence variation constrain pilus assembly to the packing of flat subunit faces against α1 helices. Helical fibre assembly is postulated to form a core of coiled α1 helices banded by β-sheet, leaving carbohydrate and hypervariable sequence regions exposed to solvent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kabsch, W., Mannherz, H. G., Suck, D., Pai, E. F. & Holmes, K. C. Nature 347, 37–43 (1990).
Schutt, C. E., Myslik, J. C., Rozycki, M. D., Goonesekere, N. C. W. & Lindberg, U. Nature 365, 810–816 (1993).
Rayment, I. et al. Science 261, 50–58 (1993).
Nogales, E., Wolf, S. G., Khan, I. A., Luduena, R. F. & Downing, K. H. Nature 375, 424–427 (1995).
Mimori, Y. et al. J. molec. Biol. 249, 69–87 (1995).
Morgan, D. G., Owen, C., Melanson, L. A. & DeRosier, D. J. molec. Biol. 249, 88–110 (1995).
Bullitt, E. & Makowski, L. Nature 373, 164–167 (1995).
Ottow, J. C. G. A. Rev. Microbiol. 29, 79–108 (1975).
Woods, D. E., Strauss, D. C., Waldermar, G. J. Jr, Berry, V. K. & Bass, J. A. Infect. Immun. 29, 1146–1151 (1980).
Buchanan, T. M. & Pierce, W. A. Infect. Immun. 13, 1483–1489 (1976).
Jonsson, A., Ilver, D., Falk, P. & Normark, S. Molec. Microbiol. 13, 403–416 (1994).
Bradley, D. E. Can. J. Microbiol. 26, 146–154 (1980).
Bradley, D. E. Virology 58, 149–163 (1974).
Dalrymple, B. & Mattick, J. S. J. molec. Evol. 25, 261–269 (1987).
Strom, M. S., Nunn, D. N. & Lory, S. Meth. Enzym. 235, 527–540 (1994).
Taniguchi, T., Fujino, Y., Yamamoto, K., Miwatani, T. & Honda, T. Infect. Immun. 63, 724–728 (1995).
Boslego, J. W. et al. Vaccine 9, 154–162 (1991).
Stewart, D. J., Clark, B. L., Peterson, J. E., Griffiths, D. A. & Smith, E. F. Res. vet. Sci. 32, 140–147 (1982).
Schoolnik, G. K. & Mietzner, T. A. in New Generation Vaccines (eds Woodrow, G. C. & Levine, M. M.) 565–597 (Dekker, New York, 1990).
Mattick, J. S., Hobbs, M., Cox, P. T. & Dalrymple, B. P. in Genetics and Molecular Biology of Anaeorobic Bacteria 517–545 (Springer, New York, 1993).
Seifert, H., Wright, C., Jerse, A., Cohen, M. & Cannon, J. J. clin. Invest. 93, 2744–2749 (1994).
Borst, P., Bitter, W., McCulloch, R., Van Leeuwen, F. & Rudenko, G. Cell 82, 1–4 (1995).
Rudel, T., Scheuerpflug, I. & Meyer, T. F. Nature 373, 357–359 (1995).
Nassif, X. et al. Proc. natn. Acad. Sci. U.S.A. 91, 3769–3773 (1994).
Mattick, J. S. et al. J. Bact. 169, 33–4l (1987).
Elleman, T. C. & Peterson, J. E. Molec. Microbiol. 1, 377–380 (1987).
Dupuy, B. & Pugsley, A. P. J. Bact. 176, 1323–1331 (1994).
Parge, H. E. et al. J. biol. Chem. 265, 2278–2285 (1990).
Orengo, C. & Thornton, J. M. Structure 1, 105–120 (1993).
Seifert, H. & So, M. Microbiol. Rev. 52, 327–336 (1984).
Hamadeh, R. M., Jarvis, G. A., Estabrook, M. & Griffiss, J. M. in Ninth Int. Pathogenic Neisseria Conf. 132–133 (SCC, Winchester, 1994).
Virji, M. et al. Molec. Microbiol. 10, 1013–1028 (1993).
Watts, T. H., Kay, C. M. & Paranchych, W. Can. J. Biochem. 60, 867–872 (1982).
Watts, T. H., Kay, C. M. & Paranchych, W. Biochemistry 22, 3640–3646 (1983).
Biswas, G. D., Sox, T., Blackman, E. & Sparling, P. F. J. Bact. 129, 983–992 (1977).
Virji, M., Heckels, J. E., Potts, W. J., Hart, C. A. & Saunders, J. R. J. gen. Microbiol. 135, 3239–3251 (1989).
Rothbard, J. B., Fernandez, R., Wang, L., Teng, N. N. H. & Schoolnik, G. K. Proc. natn. Acad. Sci. U.S.A. 82, 915–919 (1985).
Folkhard, W., Marvin, D. A., Watts, T. H. & Paranchych, W. J. molec. Biol. 149, 79–93 (1981).
Watts, T. H., Scraba, D. G. & Paranchych, W. J. Bact. 151, 1508–1513 (1982).
Pasloske, B. L., Scraba, D. G. & Paranchych, W. J. Bact. 171, 2142–2147 (1989).
McRee, D. E. J. molec. Graph. 10, 44–47 (1992).
Furey, W. & Swaminathan, S. in ACA Meeting Abstr. 73 (Am. Crystallogr. Assoc., New York, 1990).
Read, R. J. Acta Crystallogr. A 42, 140–149 (1986).
Brünger, A. T. X-PLOR: A System for X-ray Crystallography and NMR (Yale Univ. Press., New Haven, 1992).
Segal, E., Billyard, E., So, M., Storzbach, S. & Meyer, T. F. Cell 40, 293–300 (1985).
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. J. appl. Crystallogr. 26, 283–291 (1993).
Carson, M. J. appl. Crystallogr. 24, 958 (1991).
Swanson, J. & Koomey, J. M. in Mobile DNA (eds Berg, D. & Howe, M.) 743–761 (Am. Soc. Microbiol., Washington DC, 1989).
Connolly, M. L. Science 221, 709–713 (1983).
Gilson, M. K. & Honig, B. Proteins: Struct. Funct. Gen. 4, 7–18 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parge, H., Forest, K., Hickey, M. et al. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 378, 32–38 (1995). https://doi.org/10.1038/378032a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/378032a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.