Abstract
We report on the analysis of a murine anaplastic lymphoid cell line TS1G6, established recently by interleukin (IL)-9 transfection. TS1G6 revealed a highly characteristic pattern of large anaplastic cells with mononuclear, binuclear, or multinuclear cells resembling Hodgkin (H) or Sternberg-Reed (SR) cells. This cell line is tumorigenous after injection of as few as 104 lymphoma cells into nude or immunocompetent C57Bl/6 mice and leads to death from progressive disease of all treated animals within a few weeks. The histological analysis of these tumors revealed a diffuse large cell malignant lymphoma that is morphologically almost identical to human anaplastic large cell lymphoma (ALCL). The lymphoma cells did not show overexpression of the anaplastic lymphoma kinase (ALK) gene, which is found in about 50% of the cases of human ALCL. Thus, this model may be an animal model for an important subset of human ALCL. The cytokine profile, which is of the T helper 2 type, showed strong parallels to the human lymphoma counterpart. Mice suffering from such lymphomas could not be cured with a regimen using high dose cyclophosphamide similar to many ALCL patients. Such an animal model for ALCL has not yet been recognized, but may provide the basis for investigating new antitumor immunotherapies in a fully immunocompetent host.
Similar content being viewed by others
Introduction
Anaplastic large cell lymphoma (ALCL) is defined by morphology with the presence of large, anaplastic, sometimes Hodgkin (H)- and Sternberg-Reed (RS)-like cells and sometimes a carcinoma-like growth pattern with intrasinusoidal spreading combined with CD30 expression. The infiltrate is accompanied by a variable admixture of histiocytes, epithelioid cells, neutrophils, eosinophils, lymphocytes, and plasma cells (Lennert and Feller, 1992). Most cases of human ALCL were shown to be of T- or 0-cell origin, whereas only a minority of cases showed an expression of B-cell differentiation antigens. Moreover, the normal cellular counterpart for anaplastic large cell lymphoma is not yet recognized (Harris et al, 1994).
Because there is major interest in defining the lymphomagenesis of ALCL, several groups have tried to establish ALCL-cell lines for many years. However, only a small number of such human cell lines currently exist (Barbey et al, 1990; Dirks et al 1996; Drexler and Minowada, 1992; Drexler, 1993). Only four in vivo models of ALCL have been reported so far (Kinney et al, 1993; Kuefer et al, 1997; Pasqualucci et al, 1995; Terenzi et al, 1996).
An animal model mimicking this kind of human lymphoma can be advantageous for the establishment of new therapeutic strategies. However, it has been shown to be difficult to establish heterotransplants of malignant lymphomas successfully (Kapp et al, 1992). Moreover, clinical trials on human lymphoma models in immunodeficient mice were hampered by the fact that these animals were devoid of a functional immune system and, thus, artificial with respect to the biology and clinical behavior of the growing tumors. In this study we report the morphology and biology of a murine anaplastic lymphoid cell line, TS1G6, and the respective tumors in syngeneic C57Bl/6 mice, which showed strong parallels to their human counterpart.
Results and Discussion
The study of the biology of ALCL has been hampered by the difficulty of establishing cell lines from such lymphoma. Until now, reports of well-characterized animal models for ALCL had not been published. In this study we describe the progressive in vivo growth of an interleukin (IL)-9 transformed T cell line (TS1G6) in immunocompetent syngeneic mice that developed ALCL-like malignant anaplastic large cell lymphoma.
Morphological and Immunohistochemical Characterization of TS1G6 Cell Line
The murine cell line TS1G6 was reported in detail earlier (Uyttenhove et al, 1991). In brief, an intra-abdominal (IAb)-restricted, CD3+, CD4+ T helper cell clone (TS1) derived from a C57Bl/6 mouse and specific for human keyhole limpet hemocyanin (KLH) was cultured without antigen and feeder cells, but in the presence of IL-9. This growth factor-dependent TS1 clone was subsequently transfected with the IL-9 cDNA in the expression vector pBMGneo (using the bovine papilloma virus promoter and neomycin as selection drug). Although the cells transfected with the sense construct no longer required exogenously added IL-9, the cells transfected with the antisense-control (IL-9 antisense construct) remained IL-9 dependent. The uncloned transfected cells were subcloned and the clones, TS1G6 and TS1E10 (sense and anti-[mis-]sense respectively), were further tested for tumorigenicity. Although all animals treated with TS1G6 cells showed development of progressive tumors, not a single tumor was obtained after injection of TS1E10 cells. The capacity to form and establish tumors of the cell lines TS1G6/TS1E10 is shown schematically in Figure 1.
Cytologically the TS1G6 cells showed marked heterogeneity in cell size with diameters ranging from 20 μm to 100 μm. Many cells were mononuclear, but bi- or multinucleated (sometimes more than 10 nuclei) cells were frequently found. Much similar to single cells derived from human ALCL, the nuclei have vesicular chromatin with one or several prominent, centrally located, sometimes inclusion-like, eosinophilic nucleoli. The cytoplasm is abundant, vacuolated, and basophilic in Pappenheim staining (Fig. 2a and b).
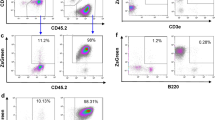
The immunophenotype of the cultured cells analyzed by FACS (fluorescence-activated cell sorting) showed a progressive loss or down-regulation of some antigens like CD3 and CD4 after long term in vitro culture (data not shown) (Renauld et al, 1993). This can also be observed in human ALCL tumors, where more than 30% of the so-called 0-cell-type show no expression of T-cell antigens at all. However, as in human ALCL (Harris et al, 1994; Louahed et al, 1995), the activation antigens CD30 and CD25 and the transferrin-receptor CD71 were expressed (Fig. 3, a to c). The hallmark antigen for human ALCL, CD30, could be demonstrated on the majority of tumor cells, although weakly. The cDNA- and protein-sequences for human and mouse CD30 differ significantly, and thus the function and expression intensity of CD30, which is not well understood even for the human system, may also differ (Kinney et al, 1993). In summary, the phenotype is well in accordance with cells derived from human ALCL of the T-cell type (Harris et al, 1994; Louahed et al, 1995).
Analysis of TCR-Rearrangement
The cloning procedure of the TS1 cells assured that the TS1G6 T-cells were derived from a single cell and thus of clonal nature. This was confirmed by analyzing the T cell receptor (TCR)-γ gene locus. We found a monoclonal rearrangement pattern (100% match with the germline sequence of TCR-γ1.1-Jγ4) (EMBL Database; EMBL, Heidelberg, Germany).
mRNA-Cytokine Profile of the Cell Line TS1G6 Using RT-PCR
To correlate the functional capacity of the lymphoma cells to one of the known T helper (TH) cell cytokine expression profiles (TH1 or TH2), we analyzed the cytokine profile of TS1G6 cells at the level of mRNA. The reverse transcriptase-polymerase chain reaction (RT-PCR) results are shown in Table 1 and Figure 4.
The cytokine mRNAs for IL-4, IL-5, IL-6, IL-9, and IL-10 were expressed, whereas the mRNAs for IL-2, IL-12, interferon-γ (IFN-γ), and granulocyte macrophage-colony stimulating factor (GM-CSF) could not be detected. Moreover, the cytokine receptors IL-7R and IL-9R were also expressed (data not shown). This cytokine expression profile displays the typical profile of TH2 cells, because the B-cell stimulating/modulating cytokines IL-4, IL-9, and IL-10 were expressed whereas the TH1 cytokines, IL-2 and interferon-γ, were not detectable (Fiorentino et al, 1989).
It is supposed that TH2 cytokines are expressed by CD30+, CD4+ T-helper cells (Del Prete et al, 1995). CD30 expression seems to be associated with the differentiation/activation pathway of TH2 cells, and CD30 triggering by CD30 ligand-expressing, antigen-presenting cells may represent an important co-stimulus for the development of TH2 responses. In contrast, Hamann et al (1996) could not find evidence for discrimination of TH1- and TH2-type T cells by CD30 expression.
Although we have not made an attempt to correlate CD30 expression with TH1- and TH2-type cytokine expression pattern of nonneoplastic T cells in our model, we could clearly show weak CD30 expression and expression of TH2 cytokines in the cell line TS1G6.
It is of interest that this pattern of cytokine expression is found in most, if not all human ALCL and even, at least in part, in many other non-Hodgkin’s lymphomas of the T cell type (Boulland et al, 1998; Merz et al, 1991a; Merz et al, 1991b).
In addition, mRNA for the cytokine receptor IL-9R was also detected. This result was expected because the cloning procedure of the mother cell line TS1 was based on the exogenous addition of IL-9 and its requirement for growth of the cells. Although we do not know whether this pattern of cytokine expression is transferred into protein expression, we suppose that this is the case because bioactive IL-9, for example, could be measured in the supernatant of the cells (measured by the proliferation of IL-9 dependent Mo7E cells; data not shown).
It is tempting to speculate about the suppression of the host immune response, eg, cytotoxic response against the ALCL tumor through expression of TH2 cytokines and subsequent down-regulation of the TH1 “cytotoxic” system. It is worthwhile to study this hypothesis in more detail because new therapeutic strategies can be developed on the basis of the counterbalancing action of the TH1/TH2 cytokine network.
Analysis of ALK1 Expression by RT-PCR and Northern-blot
Aberrant expression of anaplastic lymphoma kinase (ALK) occurs in 30% to 60% of human anaplastic large cell non-Hodgkin’s lymphomas of the T or 0 cell type. By using RT-PCR, we were not able to detect mRNA for the murine homolog of human ALK. As a positive control tissue, mouse brain tissue was used in which we could detect strong expression of murine ALK by using RT-PCR and northern blot analysis. Because approximately 50% of human ALCLs were negative for ALK, the overexpression or aberrant expression of other oncogenes, or the loss of suppressor genes, may also be relevant for the transformation of T lymphocytes into anaplastic lymphoma cells (Falini et al, 1999; Gascoyne et al, 1999).
Subcutaneous and Disseminated Growth after Injection of TS1G6 Cells into Syngeneic C57Bl/6 Mice
Subcutaneous (sc) or intraperitoneal (ip) injection of cells from the cell line TS1G6 into C57BI/6 mice led to in vivo growth of malignant lymphomas. The sc or ip injection of as few as 104 to 106 of these transfected TS1G6 cells into syngeneic C57Bl/6 mice resulted in the growth of malignant lymphoma in all animals (100%, 20/20), whereas the TS1E10 cells (transfected with antisense construct) were still dependent on IL-9 in culture and never produced tumors in syngeneic mice nor in immunodeficient nude mice.
Tumors were visible after 9 to 12 days when 106 cells were injected, after 12 to 14 days when 105 cells were injected, and after 14 to 16 days when 104 cells were injected. A typical growth curve of these tumors is shown in Figure 5.
Signs of progressive disease became visible approximately 4 to 8 weeks after the injection of TS1G6 cells into syngeneic mice. Because the tumors grew slightly faster when cells were injected into nude mice instead of syngeneic mice (Fig. 6), we suppose that the immune system is able to recognize the tumor cells on the basis of tumor rejection antigens and that T cells are necessary for this recognition and for induction of an antitumor response. Such an antitumor response, which is of little relevance for the velocity of tumor growth or for the outcome of the animals in untreated groups, may be boosted by immunotherapeutic strategies and, thus, may be important for tumor rejection.
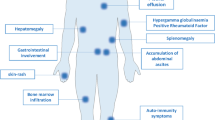
Animals developed first local tumors, either in the skin and subcutaneous tissue or in the abdomen, depending on the route of injection. From there, dissemination takes place per continuitatem or via lymphatics. The animals developed malignant lymphomas in nearly all lymph nodes and in the spleen. The axillary, inguinal, cervical, paraaortic, caecal, and renal lymph nodes were involved (Fig. 7). In addition, extranodal growth was observed in the liver, the kidneys, and the lungs. When animals showed heavy tumor burden (up to 10g total tumor weight), some lymph nodes were about 1 cm in size, and the ratio of total tumor to total body weight was up to 1 to 3, whereas healthy female C57Bl/6 mice weigh approximately 20 to 22g in this age group. Later, the animals often showed tumor cachexia. When the local tumors grew to a considerable size, or when the animals showed signs of distress, they were killed.
There was no evidence of involvement of the bone marrow or thymus. However, because the animals were killed when they suffered from visible tumor burden, we could not exclude that tumors could also spread to these organs.
Histology of the TS1G6 Tumors
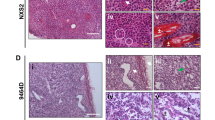
Histopathological examinations of the local tumor demonstrated tumors with large, bizarre, anaplastic cells, some of which in part resemble H or SR cells (Fig. 8). All tumors showed, at least in part, a cohesive growth pattern and were accompanied by a discrete infiltration of neutrophils and/or eosinophils. Some tumors also showed diffuse fibrosis. The cytology of the tumor cells was identical to that of human ALCL, as shown by the fact that three expert pathologists diagnosed ALCL independently not knowing that they were dealing with a murine lymphoma.
The local skin tumors, as in human ALCL, had a tendency to exulcerate, whereby the tumor cells were arranged in tight tumor nodules infiltrating first the dermis and then the underlying muscles or soft tissues. The local tumors spread per continuitatem, thereby infiltrating the entire abdominal wall and leading to peritoneal dissemination. However, in some animals the nodal and hematogenous dissemination preceded the intraperitoneal dissemination that had resulted from spreading per continuitatem. It is of special interest that the pancreatic gland was frequently infiltrated. This is also sometimes found in human ALCL.
Enlarged lymph nodes showed obliteration of their architecture. Primarily, they had been infiltrated by the tumor cells via the sinuses (Fig. 9a), leading to subsequent infiltration of the T-zones (Fig. 9b) and finally complete destruction of the lymph node. Hematogenous metastases were found in the vessels of the liver and the lung; some of these vessels were occluded by tumor cell thrombi.
In summary, the tumors show strong parallels, both in their biological behavior and in cytology and histology, to human ALCL. The most important analogies are reviewed in Table 2.
Treatment with High-Dose Cyclophosphamide
Mice with a local growth of TS1G6 tumors of 0.4 cm in diameter were treated with the chemotherapeutic drug cyclophosphamide (which is used as an important chemotherapeutic drug in the treatment of human ALCLs). A dose of 125 mg cyclophosphamide/kg body weight, equivalent to a high-dose regimen in humans, was injected intraperitoneally and administered as often as six times in intervals as short as 10 days. We have analyzed the blood parameters of the animals after treatment and found complete reconstitution after 7 to 8 days. However, the shortest interval of drug delivery which was able to be tolerated by the animals was 10 days (data not shown). Six out of six mice (100%) did not show significant tumor regression, and all animals developed progressive tumors with little retardation of tumor growth compared to control animals. After increasing the dose to 250 mg/kg body weight (six times after each 10 days), six out of six mice (100%) showed significant delay of tumor growth, and some animals showed macroscopically a short phase of complete remission. However, all animals died of progressive disease (Fig. 10). Thus, the lymphoma cannot be cured by cyclophosphamide, but it is moderately sensitive to chemotherapy.
It is of particular interest, that chemotherapy, applied even in a high-dose regimen, is able to cure only about one half of human patients.
Thus, ALCL proves to be fatal in many instances, especially in those in which patients primarily present with widespread disease (Kapp et al, 1992; Kuefer et al, 1997; Purtilo et al, 1991). Therefore, there is a need for animal models for establishing new therapeutical agents or treatment modalities that can cure patients who cannot be cured by already existing therapies and that may be not as harmful as the standard therapies. Until recently, testing of new therapeutic agents, eg, chemo- or immunotherapeutical drugs, has relied extensively upon mice for in vivo preclinical studies (Dick et al, 1991; McCune et al, 1991; Mosier, 1991; Purtilo et al, 1991). Unfortunately, animal models of malignant lymphomas are rarely reported, and there are only few reports on ALCL (Pasqualucci et al, 1995; Terenzi et al, 1996; Tian et al, 1995). Furthermore, these animal models do not always mimic the physiological and pathophysiological processes that take place in humans. The failure to cure many patients suffering from ALCL is paralleled by the TS1G6 mouse model.
In summary, we have presented a mouse lymphoma model for a subset of human ALCL (cases without ALK1-expression), which provides the basis for overcoming many of the above-mentioned limitations of conventional therapy and allows further study of the biology and pathogenesis of ALCL in the immunocompetent host. Moreover, it will be interesting to find out whether a TH2 cytokine profile will hold true also in human ALCL. If so, this finding can be used to counterbalance the deranged and biased cytokine milieu as therapeutic strategy using cytokines or anticytokine antibodies. As shown here for the murine model, it is tempting to speculate about autocrine loops in lymphomas that may be disrupted by antisense or anticytokine treatment strategies.
Materials and Methods
In Vitro Culture of the Cell Line TS1G6 and Morphological and Enzyme Histochemical Analysis of Cultured Cells
The cell line TS1G6 was maintained in RPMI 1640 supplemented with 10% heat inactivated fetal calf serum, 2 mmol/L L-glutamine, 50 IU/mL penicillin, and 50 μg/mL streptomycin. The cells were cultured in an atmosphere consisting of 5% CO2 and humidified air.
The cells were prepared for morphological and enzyme-histochemical analysis using ficoll-gradient separation (Ficoll-Hypaque; Pharmacia Biotechnology, Freiburg, Germany). Cytospin preparations were prepared with 100,000 cells per slide. Slides were stained with Pappenheim (May-Grünwald-Giemsa), hematoxylin-eosin, nonspecific esterase, myeloperoxidase, alkaline phosphatase, Naphthol AS-D chloroacetate esterase, periodic acid-Schiff reaction, and acid phosphatase according to standard histochemical/enzyme-histochemical procedures.
Flow Cytometric Analysis
The TS1G6 expression of CD30, CD25, CD71, CD3, CD8, and CD4 was evaluated by using the matched murine monoclonal antibody reagents directly conjugated to phycoerythrin (PE) or fluorescein isothiocyanate (FITC) (Pharmingen, Heidelberg, Germany). Cultured Cells were washed with PBS, centrifuged at 400 ×g for 5 minutes and resuspended at 1 × 105 to 1 × 106 in 200 μl PBS/tube. Cells were incubated using 20 μl of PE- or FITC-conjugated anti-mouse antibody reagent including blocking solution (bovine serum albumin, 10 mg/ml; nonconjugated rabbit anti-mouse antibodies, 10 mg/ml) for 20 minutes (in darkness at room temperature) and were washed. After resuspending in 300 μl PBS, cells were analyzed within 20 minutes on a FACScan cell analyzer (Becton Dickinson, Heidelberg, Germany) using fluorescence 1 (FL-1) or fluorescence 2 (FL-2) for specific immunolabeling. The Becton Dickinson Cellquest program was used for dual- and single-color analysis respectively. Cell debris were eliminated from the analysis using a gate on forward and sideward scatters. Quadrant markers were set using negative isotype matched controls to define the limits of nonspecific staining.
Expression of mRNA Species for Cytokines/Cytokine-Receptors by RT-PCR
The cytokine/cytokine-receptor mRNA expression profile of TS1G6 cells was obtained by RT-PCR analysis, which was performed using reverse-transcribed cDNA from purified, total TS1G6 cell RNA and standard procedures (Ausubel and Frederick, 1999). First-strand cDNAs were prepared after checking RNA integrity by agarose-gel electrophoresis and ethidium bromide staining. Total cellular RNA (5 μg) was used for reverse transcription at 37°C for 60 minutes in total volume of 50 μl containing 0.5 μg oligo-dT (12–18 mer) (Pharmacia, Freiburg, Germany), 1 mmol/L dithiothreitol, 0.5 mmol/L dNTP, 100 U of RNAse inhibitor (RNAsin) and 50 U of recombinant murine Moloney-leukemia-virus (MMLV) reverse transcriptase (BRL Life Sciences, Gaithersburg, Maryland). The β-actin PCR was used again for control of high quality, first-strand synthesis. After an initial “hot start” at 94°C for 2 minutes, 30 cycles (94°C for 40 seconds, 60°C for 45 seconds, 72°C for 2 minutes) were performed in a thermocycler (Biometra GmbH, Goettingen, Germany). Gel analysis of PCR products was used to compare the amount of PCR-amplified products (eg, cytokines) produced after different numbers of amplification cycles (24, 27, and 30 cycles).
Primer sequences used for PCR were the following: mouse β-actin sense primer (5′-tgacgaggcccagagcaagagag-3′), mouse β-actin antisense primer (5′-ctaggagccagagcagtaatctc-3′), mouse IL-2 sense (5′-atgtacagcatgcagctcgcatc-3′), mouse IL-2 antisense (5′-ggcttgttgagatgatgctttgaca-3′), mouse IL-4 sense (5′-atgggtctcaacccccagctagt-3′), mouse IL-4 antisense (5′-gctctttaggctttccaggaagtc-3′), mouse IL-5 sense (5′-atgagaaggatgcttctgcacttga-3′), mouse IL-6 sense (5′-atgaagttcctctctgcaagagact-3′), mouse IL-6 antisense (5′-cactaggtttgccgagtagatctc-3′), mouse IL-9 sense (5′-cccggccgcatgttggtgacatacatccttgcc-3′), mouse IL-9 antisense (5′-ggggatccttcatggtcggcttttcagcctttt-3′), mouse IL-10 sense (5′-gcctgctcttactgactggcat-3′), mouse IL-10 antisense (5′-tcattttgatcatcatgtatgctt-3′), mouse IL-12 sense (5′-aaagtctgccggctatccagaca-3′), mouse IL-12 antisense (5′-tcaggcggagctcagatagcccat-3′), mouse GM-CSF sense (5′-gccaccatgtggctgcagaatttacttttc-3′), mouse GM-CSF antisense (5′-gtctgagaagctggattcagagct-3′), mouse INF-γ sense (5′-gctgttactgccacggcacagtc-3′) and mouse INF-γ antisense (5′-gcaacagctggtggaccactcg-3′).
Primer sequences were obtained from the EMBL sequence database (EMBL).
Northern Blot
Total RNA was isolated from mouse tissues (spleen, lymph-node, liver, and brain) and cell line TS1G6 by the method of Chomczynski and Sacchi (1987). Poly (A)+-RNA was isolated according to the instructions of the manufacturer (Quiagen, Hilden, Germany). In vitro transcription of DIG-labeled RNA probes was performed using the In Vitro Transkription Kit (Roche, Mannheim, Germany) with the PCR-generated template of mouse ALK. Primer sequences used for cloning of mouse ALK were deduced from the EMBL sequence database (EMBL) as follows: accession number of mouse mRNA for tyrosin kinase (ALK), D83002 (Iwahara et al, 1997); ALK-PCR product from position 621 to position 1398; ALK sense primer, 5′ctt cgt tgt acc ctc gct ctt ccg c-3′; and ALK antisense primer, 5′aga atg ctc tgc ctc tgg ccc atc c 3′. Northern blot was carried out using standard methods with a hybridization temperature of 68° C. Chemiluminescent detection of DIG-labeled probes was carried out as described by the manufactures with CDPstar detection (Roche).
Analysis of TCR Rearrangements
Total cellular DNA was prepared from the cell line TS1G6. Pellets of 1×106 cells were resuspended in 50 μl of proteinase K-buffer (10 mm Tris, pH 8.3, 5 0 mm KCl, 2.5 mm MgCl2) and incubated for 2 hours at 56°C in the presence of proteinase K (100 μg/ml). After inactivation of proteinase K, 1 to 5 μl (400 ng DNA) of the proceeding suspension were amplified with a Biometra DNA thermal cycler for 35 cycles (40 seconds 95°C, 45 seconds at 55°C, and 1 minute at 72°C) in a volume of 50 μl. The reaction mixture included 50 pmol of each primer, 0.2 mm of each dNTP, 50 mm KG, 10 mm Tris-HG (pH 8.3), 1.5 mm MgCl2, 0.01% gelatin, and 1.0 U of Taq polymerase (Biometra). Primer pairs used were as follows: Vγl.2: 5′gtgcaaatatcctgtatagtt-3′ and Jγ2: 5′-acagtagtaggtggcttcac-3′; Vγ1.1: Ygagagtgcgcaaatatcctgtata-3′ and Jγ4:5′-tgggggaattactacgagct-3′; Vγ2/4: 5′tatgtccttgcaacccctac-3′ and Jγ1: 5′-atgagcttagttccttctgc-3′; Vγ5/7: Y-gMatgaaggcccggaca-3′ and Jγ1; Vγ4/6: 5′-acaagtgttcagaagcccga-3′ and Jγ1; Vγ3/5: 5′-tggatatctcaggatcagct-3′ and Jγ1. Samples (5 μl) were electrophoresed in 2% agarose-gel and visualized by ethidium-bromide staining.
Animals
Female 6- to 10-week-old mice of the strain C57Bl/6 were purchased from Charles River Inc. (Sulzfeld, Germany). Five to nine animals were kept in each cage. Animals were exposed to 12 hours light/12 hours dark cycles, and standard food and water were provided as needed.
For studying tumor growth in T cell deficient mice, 6-week-old CD1 nuBR mice, obtained from Charles River Inc. were used. They were kept in isolation under strictly controlled, specific pathogen-free conditions.
Establishment of an In Vivo Tumor Model in C57B/l6 Mice by Transplantation of the Cell Line TS1G6
TS1G6 cells (1 × 104, 105, or 106), growing exponentially, were injected into the right flank of the mice either subcutaneously or intraperitoneally after having been resuspended in 200 μl PBS. The animals were killed when a tumor of more than 1.5 cm in diameter was palpable, or when they presented with a detectable abdominal swelling, or when their health deteriorated.
Treatment of Animals
Treatment of tumor-bearing mice with high-dose cyclophosphamide was administered six times in intervals of 10 days, with therapy starting when local growth of TS1G6 tumors was as small as 0.4 cm in diameter. Doses of 125 mg and 250 mg cyclophosphamide/mg body weight were injected intraperitoneally. Cyclophosphamide was a product of ASTA Medica AWD GmbH (Frankfurt, Germany).
Histopathology and Immunhistology of TS1G6 Tumors
The material for microscopic analysis was taken during necropsy after ether anesthesia and cervical dislocation. Skin, lymph node, bone marrow, liver, spleen, and lung tissues were dissected, fixed, paraffin-embedded, and stained for hematoxylin-eosin, Giemsa’s solution, PAS, and Gomori’s silver stain according to standard techniques.
Accession codes
References
Ausubel I and Frederick M (1999). Preparation and analysis of RNA. In: Current Protocols in Molecular Biology, (vol 1). New York: John Wiley and Sons, 4.0.1–4.10.11.
Barbey S, Gogusev J, Mouly H, Le-Pelletier O, Smith W, Richard S, Soulie J, and Nezelof C (1990). DEL cell line: A “malignant histiocytosis” CD30+ t(5;6)(q35;p21) cell line. Int J Cancer 45: 546–553.
Boulland ML, Meignin V, Leroy-Viard K, Copie-Bergman C, Briere J, Touitou R, Kanavaros P, and Gaulard P (1998). Human interleukin-10 expression in T/natural killer-cell lymphomas: Association with anaplastic large cell lymphomas and nasal natural killer-cell lymphomas. Am J Pathol 153 (4):1229–1237.
Chomczynski P and Sacchi N (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159.
Del Prete G, De Carli M, D’Elios MM, Daniel KC, Almerigogna F, Alderson M, Smith CA, Thomas E, and Romagnani S (1995). CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med 182: 1655–1661.
Dick JE, Lapidot T, and Pflumio F (1991). Transplantation of normal and leukemic human bone marrow into immune-deficient mice: Development of animal models for human hematopoiesis. Immunol Rev 124: 25–43.
Dirks WG, Zaborski M, Jager K, Challier C, Shiota M, Quentmeier H, and Drexler HG (1996). The (2;5) (p23;q35) translocation in cell lines derived from malignant lymphomas: Absence of t(2;5) in Hodgkin-analogous cell lines. Leukemia 10: 142–149.
Drexler HG and Minowada J (1992). Hodgkin’s disease derived cell lines: A review. Hum Cell 5: 42–53.
Drexler HG (1993). Recent results on the biology of Hodgkin and Reed-Sternberg cells. II. Continuous cell lines. Leuk Lymphoma 9: 1–25.
Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, Verhoef G, Menestina F, Todeschini G, Paulli M, Lazzarino M, Giardini R, Aiello A, Foss HD, Araujo I, Fizzotti M, Pelicci PG, Flenghi L, Martelli MF, and Santucci A (1999). ALK+ lymphoma: Clinico-pathological findings and outcome. Blood 93: 2697–2706.
Fiorentino DF, Bond MW, and Mosmann TR (1989). Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170: 2081–2095.
Gascoyne RD, Aoun P, Wu D, Chhanabhai M, Skinnider BF, Greiner TC, Morris SW, Connors JM, Vose JM, Viswanatha DS, Coldman A, and Weisenburger DD (1999). Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 93: 3913–3921.
Hamann D, Hilkens CM, Grogan JL, Lens SM, Kapsenberg ML, Yazdanbakhsh M, and van Lier RA (1996). CD30 expression does not discriminate between human Th1- and Th2-type T cells. J Immunol 156: 1387–1391.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, and Gatter KC (1994). A revised European-American classification of lymphoid neplasms: A proposal from the International Lymphoma Study Group. Blood 84: 1361–1392.
Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, and Yamamoto T (1997). Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14: 439–449.
Kapp U, Wolf J, von Kalle C, Stein H, Fonatsch C, Schell-Fredrick E, and Diehl V (1992). Recent efforts to establish an in vivo model as a new experimental tool in the study of Hodgkin’s disease. Eur J Cancer 28A: 1408–1411.
Kinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, and Kadin ME (1993). A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am J Surg Pathol 17: 859–868.
Kuefer MU, Look AT, Pulford K, Behm FG, Pattengale PK, Mason DY, and Morris SW (1997). Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 90: 2901–2910.
Lennert K and Feller AC (1992). Histopathology of non-Hodgkin lymphomas: (based on the updated Kiel classification), 2nd ed. Springer-Verlag, Berlin: 229–244.
Louahed J, Kermouni A, Van Snick J, and Renauld JC (1995). IL-9 induces expression of granzymes and high-affinity IgE receptor in murine T-cells. J Immunol 154: 5061–5070.
McCune J, Kaneshima H, Krowska J, Namikawa R, Outzen H, Peault B, Rabin L, Shih CC, Yee E, and Lieberman M (1991). The SCID-hu mouse: A small animal model for HIV infection and pathogenesis. Annu Rev Immunol 9: 399–429.
Merz H, Fliedner A, Orscheschek K, Binder T, Sebald W, Mueller-Hermelink HK, and Feller AC (1991a). Cytokine expression in T-cell lymphomas and Hodgkinís disease. Its possible implication in autocrine or paracrine production as a potential basis for neoplastic growth. Am J Pathol 139: 1173–1180.
Merz H, Houssiau FA, Orscheschek K, Renauld JC, Fliedner A, Herin M, Noel H, Kadin M, Mueller-Hermelink HK, Van-Snick J ., and Feller AC (1991b). Interleukin-9 expression in human malignant lymphomas: Unique association with Hodgkin’s disease and large cell anaplastic lymphoma. Blood 78: 1311–1317.
Mosier DE (1991). Adoptive transfer of human lymphoid cells to severely immunodeficient mice. Models for normal human immune function, autoimmunity, lymphomagenesis, and AIDS. Adv Immunol 50: 303–325.
Pasqualucci L, Waski M, Teicher BA, Flenghi L, Bolognesie A, Stirpe F, Polito L, Falini B, and Kadin ME (1995). Antitumor activity of anti-CD30 immunotoxin (Ber-H2/saporin) in vitro and in severe combined immunodeficiency disease mice xenografted with human CD30+ anaplastic large-cell lymphoma. Blood 85: 2139–2146.
Purtilo DT, Falk K, Pirruccello SJ, Nakamine H, Kleveland K, Davis JR, Okano M, Taguchi Y, Sanger WG, and Beisel KW (1991). SCID mouse model of Epstein-Barr virus-induced lymphomagenesis of immunodeficient humans. Int J Cancer 47: 510–517.
Renauld JC, Houssiau F, Louahed J, Vink A, Van Snick J, and Uyttenhove C (1993). Interleukin-9. Adv Immunol 54: 79–97.
Terenzi A, Bolognesi A, Pasqualucci L, Flenghi L, Pileri S, Stein H, Kadin M, Bigerna B, Polito L, Tazzari PL, Martelli MF, Stirpe F, and Falini B (1996). Anti-CD30 (BER-H2) immunotoxins containing the type-1 ribosome-inactivating proteins momordin and PAP-S (pokeweed antiviral protein from seeds) display powerful antitumor activity against CD30+ tumor cells in vitro and in SCID mice. Br J Haematol 92: 872–879.
Tian ZG, Longo DL, Funakoshi S, Asai O, Ferris DK, Widmer M, and Murphy WJ (1995). In vivo antitumor effects of unconjugated CD30 monoclonal antibodies on human anaplastic large-cell lymphoma xenografts. Cancer Res 55: 5335–5341.
Uyttenhove C, Druez C, Renauld JC, Herin M, Noel H, and Van Snick J (1991). Autonomous growth and tumorigenicity induced by P40/interleukin 9 cDNA transfection of a mouse P40-dependent T cell line. J Exp Med 173: 519–522.
Author information
Authors and Affiliations
Additional information
This study was supported by a grant from Forschungsförderungsprogramm der Medizinischen Fakultät Lübeck to HM and GW (“Etablierung neuer Behandlungsstrategien des grosszellig anaplastischen Lymphoms an einem Maus-Tiermodell”) and from Amgen to HM and GW (“Forschungsprojekt zur Hyperthermie und präklinische Forschungsaktivitäten”).
Rights and permissions
About this article
Cite this article
Bittner, C., Feller, A., Renauld, J. et al. An Animal Model for Anaplastic Large Cell Lymphoma in the Immunocompetent Syngeneic C57Bl/6 Mouse. Lab Invest 80, 1523–1531 (2000). https://doi.org/10.1038/labinvest.3780162
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780162