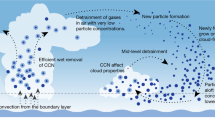
Abstract
The ‘indirect’ radiative cooling of climate due to the role of anthropogenic aerosols in cloud droplet formation processes (which affect cloud albedo) is potentially large, up to −1.5 W m−2 (ref. 1). It is important to be able to determine the number concentration of cloud droplets to within a few per cent, as radiative forcing as a result of clouds is very sensitive to changes in this quantity2, but empirical approaches are problematic3,4,5. The initial growth of a subset of particles known as cloud condensation nuclei and their subsequent ‘activation’ to form droplets are generally calculated with the assumption that cloud droplet activation occurs as an equilibrium process described by classical Köhler theory6,7. Here we show that this assumption can be invalid under certain realistic conditions. We conclude that the poor empirical correlation between cloud droplet and cloud condensation nuclei concentrations is partly a result of kinetically limited growth before droplet activation occurs. Ignoring these considerations in calculations of total cloud radiative forcing based on cloud condensation nuclei concentrations could lead to errors that are of the same order of magnitude as the total anthropogenic greenhouse-gas radiative forcing1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Intergovernmental Panel on Climate Change Climate Change 1995: The Science of Climate Change (eds Houghton, J. T. et al.) 3–7 (Cambridge Univ. Press, Cambridge, (1996)).
Charlson, R. J. et al. Climate forcing by anthropogenic aerosols. Science 255, 423–430 (1992).
Twomey, S. & Warner, J. Comparison of measurements of cloud droplets and cloud nuclei. J. Atmos. Sci. 24, 702–703 (1967).
Hegg, D. A., Ferek, R. J. & Hobbs, P. V. Light-scattering and cloud condensation nucleus activity of sulfate aerosol measured over the Northeast Atlantic Ocean. J. Geophys. Res. 98, 14887–14894 (1993).
Pueschel, R. F. et al. Aerosols in polluted versus nonpolluted air masses — long-range transport and effects on clouds. J. Clim. Appl. Meteorol. 25, 1908–1917 (1986).
Schwartz, S. E. et al. in Aerosol Forcing of Climate (eds Charlson, R. J. & Heintzenberg, J.) 251–280 (Wiley, Chichester, (1995)).
Ghan, S. J. et al. Aparameterization of cloud droplet nucleation part I: single aerosol type. Atmos. Res. 30, 197–221 (1993).
Seinfeld, J. H. Atmospheric Chemistry and Physics of Air Pollution (Wiley, New York, (1986)).
Jensen, J. B. & Charlson, R. J. On the efficiency of nucleation scavenging. Tellus B36, 367–375 (1984).
Pruppacher, H. R. & Klett, J. D. Microphysics of Clouds and Precipitation (Reidel, Dordrecht, (1978)).
Fukuta, N. & Walter, A. Kinetics of hydrometeor growth from a vapor-spherical model. J. Atmos. Sci. 27, 1160–1172 (1970).
Hobbs, P. V. in Aerosol–Cloud–Climate Interactions (ed. Hobbs, P. V.) 33–37 (Academic, San Diego, (1993)).
Mozurkewich, M. Aerosol growth and the condensation coefficient for water: a review. Aerosol Sci. Technol. 5, 223–236 (1986).
Novakov, T. & Penner, J. E. Large contribution of organic aerosols to cloud-condensation-nuclei concentrations. Nature 365, 823–826 (1993).
Rivera-Carpio, C. A. et al. Derivation of contributions of sulfate and carbonaceous aerosols to cloud condensation nuclei from mass size distributions. J. Geophys. Res. 101, 19483–19493 (1996).
Shulman, M. L. et al. Dissolution behavior and surface tension effects of organic compounds in nucleating cloud droplets. Geophys. Res. Lett. 23, 227–280 (1996).
Stumm, W. & Morgan, J. J. Aquatic Chemistry (Wiley, New York, (1981)).
Barnes, G. T. & La Mer, V. K. in Retardation of Evaporation by Monolayers: Transport Processes (ed. La Mer, V. K.) 9–33 (Academic, New York, (1962)).
Bigg, E. K. Discrepancy between observation and prediction of concentrations of cloud condensation nuclei. Atmos. Res. 20, 82–86 (1986).
Acknowledgements
This work was supported by the Office of Naval Research and the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chuang, P., Charlson, R. & Seinfeld, J. Kinetic limitations on droplet formation in clouds. Nature 390, 594–596 (1997). https://doi.org/10.1038/37576
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/37576
This article is cited by
-
Evaporation coefficient and condensation coefficient of vapor under high gas pressure conditions
Scientific Reports (2020)
-
Effect of Na and Cl ions on water evaporation on graphene oxide
Nuclear Science and Techniques (2019)
-
The test freezing temperature of C2–C6 dicarboxylic acid: The important indicator for ice nucleation processes
Science Bulletin (2008)
-
Clouds and climate
Nature (1999)
-
Cloud albedo enhancement by surface-active organic solutes in growing droplets
Nature (1999)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.