Abstract
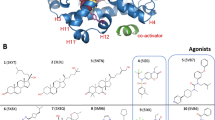
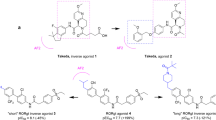
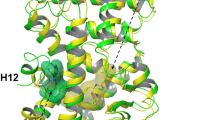
The crystal structure of the human retinoid-X receptor RXR-α ligand-binding domain reveals a previously undiscovered fold of an antiparallel α-helical sandwich, packed as dimeric units. Two helices and one loop form the homodimerization surface, and hydrophobic heptad repeats participate in stabilizing the fold. The existence of a ligand-binding pocket is proposed that would allow 9-cis retinoic acid to interact with different functional modules, including the AF-2 activating domain. Several lines of evidence indicate that the overall structure is a prototype fold of ligand-binding domains of nuclear receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Leid, M., Kastner, P. & Chambon, P. Trends biochem. Sci. 17, 427–433 (1992).
Chambon, P. Semin. Cell Biol. 5, 115–125 (1994).
Zechel, C. et al. EMBO J. 13, 1425–1433 (1994).
Kurokawa, R. et al. Nature 371, 528–531 (1994).
Giguère, V. Endocrine Rev. 15, 61–79 (1994).
Green, S. & Chambon, P. Trends Genet. 4, 309–314 (1988).
Evans, R. M. Science, 240, 889–895 (1988).
Gronemeyer, H. A. Rev. Genet. 25, 89–123 (1991).
Laudet, V., Hanni, C., Coll, J., Catzeflis, F. & Stehelin, D. EMBO J. 11, 1003–1013 (1992).
Mangelsdorf, D. J., Ong, E. S., Dyck, J. A. & Evans, R. M. Nature 345, 224–229 (1990).
Härd, T. et al. Science 248, 157–160 (1990).
Luisi, B. F. et al. Nature 352, 497–505 (1991).
Schwabe, J. W. R., Chapman, L., Finch, J. T. & Rhodes, D. Cell 75, 567–578 (1993).
Lee, M. S., Kliewer, S. A., Provencal, J., Wright, P. E. & Evans, R. M. Science 269, 1117–1121 (1993).
Schiltz, M., Prangé, T. & Fourme, R. J. appl. Cryst. 27, 950–960 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Brünger, A. T., Kuriyan, J. & Karplus, M. Science 235, 458–460 (1987).
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. J. appl. Crystallogr. 26, 283–291 (1993).
Janin, J., Miller, S. & Chothia, C. J. molec. Biol. 204, 155–164 (1988).
Zhang, X. K., Salbert, G., Lee, M. O. & Pfahl, M. Molec. cell. Biol. 14, 4311–4323 (1994).
Leid, M. et al. Cell 68, 377–395 (1992).
Au-Fliegner, M., Helmer, E., Casanova, J., Raaka, B. M. & Samuels, H. H. Molec. cell. Biol. 13, 5725–5737 (1993).
Forman, B. M. & Samuels, H. H. Molec. Endocr. 90, 1293–1301 (1990).
Leng, X. et al. Molec. cell. Biol. 15, 255–263 (1995).
Newcomer, M. E. Structure 1, 7–18 (1993).
Reese, J. C., Wooge, C. H. & Katzenellenbogen, B. S. Molec. Endocr. 6, 2160–2166 (1992).
Carlstedt-Duke, J. et al. J. biol. Chem. 263, 6842–6848 (1988).
Strömstedt, P. E., Berkenstam, A., Jörnvall, H., Gustafsson, J. A. & Carlstedt-Duke, J. J. biol. Chem. 265, 12973–12977 (1990).
Koelle, M. R. et al. Cell 67, 59–77 (1991).
Fawell, S. E., Lees, J. A., White, R. & Parker, M. G. Cell 60, 953–962 (1990).
Veldscholte, J. et al. Biochem. biophys. Res. Commun. 173, 534–540 (1990).
Chakraborti, P. K., Garabedian, M. J., Yamamoto, K. R. & Simons, S. S. J. biol. Chem. 266, 22075–22078 (1991).
Wrenn, C. K. & Katzenellenbogen, B. S. J. biol. Chem. 268, 24089–24098 (1993).
Sakurai, A. et al. Proc. natn. Acad. Sci. U.S.A. 86, 8977–8981 (1989).
Durand, B. et al. EMBO J. 13, 5370–5382 (1994).
Nagpal, S., Friant, S., Nakshatri, H. & Chambon, P. EMBO J. 12, 2349–2360 (1993).
Zenke, M., Munoz, A., Sap, J., Vennström, B. & Beug, H. Cell 61, 1035–1049 (1990).
Danielian, P. S., White, R., Lees, J. A. & Parker, M. G. EMBO J. 11, 1025–1033 (1992).
Barettino, D., Vivanco Ruiz, M. d. M. & Stunnenberg, H. G. EMBO J. 13, 3039–3049 (1994).
Le Douarin, B. et al. EMBO J. (in the press).
Meyer, M. E. et al. Cell 57, 433–442 (1989).
Tasset, D., Tora, L., Fromental, C., Scheer, E. & Chambon, P. Cell 62, 1177–1187 (1990).
Yao, T. P., Segraves, W. A., Oro, A. E., McKeown, M. & Evans, R. M. Cell 71, 63–72 (1992).
Brünger, A. T. Nature 355, 472–475 (1992).
Kabsch, W. J. appl. Crystallogr. 21, 916–924 (1988).
Collaborative Computational Project no. 4 Acta crystallogr. D50, 760–763 (1994).
Arnez, J. G. J. appl. Crystallogr. 27, 649–653 (1994).
Nicholls, A., Sharp, K. A. & Honig, B. Proteins 11, 281–286 (1991).
Merritt, E. A. & Murphy, M. E. P. Acta crystallogr. D50, 869–873 (1994).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bourguet, W., Ruff, M., Chambon, P. et al. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-α. Nature 375, 377–382 (1995). https://doi.org/10.1038/375377a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/375377a0
This article is cited by
-
A mechanism-based understanding of altered drug pharmacokinetics by gut microbiota
Journal of Pharmaceutical Investigation (2023)
-
Retinoid X Receptor: Cellular and Biochemical Roles of Nuclear Receptor with a Focus on Neuropathological Involvement
Molecular Neurobiology (2022)
-
Overview of the structure-based non-genomic effects of the nuclear receptor RXRα
Cellular & Molecular Biology Letters (2018)
-
Defining a conformational ensemble that directs activation of PPARγ
Nature Communications (2018)
-
Molecular dynamics simulation of human estrogen receptor free and bound to morpholine ether benzophenone inhibitor
Theoretical Chemistry Accounts (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.