Abstract
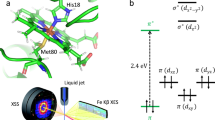
MYOGLOBIN is a globular haem protein that reversibly binds lig-ands such as O2 and CO. Single photons of visible light can break the covalent bond between CO and the haem iron in carbonmonoxy-myoglobin (MbCO) and thus form an unstable intermediate, Mb*CO, with the CO inside the protein1,2. The ensuing rebinding process has been extensively studied as a model for the interplay of dynamics, structure and function in protein reactions. We have used X-ray crystallography at liquid-helium temperatures to determine the structure of Mb*CO to a resolution of 1.5 Å. The photodissociated CO lies on top of the haem pyrrole ring C. Comparison with the CO-bound and unligated myoglobin structures reveals that on photodissociation of the CO, the haem 'domes', the iron moves partially out of the haem plane, the iron–proximal histidine bond is compressed, the F helix is strained and the distal histidine swings towards the outside of the ligand-binding pocket.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Antonini, E. & Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands (North-Holland, Amsterdam, 1971).
Austin, R. H., Beeson, K. W., Eisenstein, L., Frauenfelder, H. & Gunsalus, I. C. Biochemistry 14, 5355–5373 (1975).
Steinbach, P. J. et al. Biochemistry 30, 3988–3999 (1991).
Ahmed, A. M. et al. Chem. Phys. 158, 329–351 (1991).
Sassaroli, M., Dasgupta, S. & Rousseau, D. L. J. biol. Chem. 261, 13704–13713 (1986).
Rousseau, D. L. & Argade, P. V. Proc. natn. Acad. Sci. U.S.A. 83, 1310–1314 (1986).
Alben, J. O. et al. Proc. natn. Acad. Sci. U.S.A. 79, 3744–3748 (1982).
Ormos, P. et al. Proc. natn. Acad. Sci. U.S.A. 85, 8492–8496 (1988).
Rothberg, L. J., Roberson, M. & Jedju, T. M. Proc. Soc. photo-opt. Instrum. Engrs 1599, 309–315 (1991).
Teng, T.-Y, Huang, H. W. & Olah, G. A. Biochemistry 26, 8066–8072 (1987).
Powers, L. et al. Biochemistry 26, 4785–4796 (1987).
Stavrov, S. S. Biophys. J. 65, 1942–1950 (1993).
Straub, J. E. & Karplus, M. Chem. Phys. 158, 221–248 (1991).
Gibson, Q. H., Regan, R., Elber, R., Olson, J. S. & Carver, T. E. J. biol. Chem. 268, 17908–17916 (1992).
Kuczera, K., Lambry, J. C., Martin, J.-L & Karplus, M. Proc. natn. Acad. Sci. U.S.A. 90, 5805–5807 (1993).
Case, D. A. & Karplus, M. J. molec. Biol. 132, 343–368 (1979).
Bartunik, H. D. Nucl. Instrum. Meth. 208, 523–533 (1983).
Phillips, G. N. Jr, Arduini, R. M., Springer, B. A. & Sligar, S. G. Proteins Struct. Funct. Genet. 7, 358–365 (1990).
Hong, M. K. thesis, Univ. Illinois at Urbana-Champaign (1989).
Ringe, D., Petsko, G. A., Kerr, D. E. & Ortiz de Montellano, P. R. Biochemistry 23, 2–4 (1984).
Dasgupta, S. & Spiro, T. G. Biochemistry 25, 5941–5948 (1986).
Teng, T.-Y. J. appl. Crystallogr. 23, 387–391 (1990).
Kabsch, W. J. appl. Crystallogr. 26, 795–800 (1993).
Brünger, A. T. X-PLOR (Version 3.1) Manual (Yale Univ., New Haven, 1993).
Agmon, N. & Hopfield, J. J. J. chem. Phys. 79, 2042–2053 (1983).
Read, R. J. Acta crystallogr. A 42, 140–149 (1986).
Quillin, M. L., Arduini, R. M., Oison, J. S. & Phillips, G. N. Jr J. molec. Biol. 234, 140–155 (1993).
Cheng, X. & Schoenborn, B. P. J. molec. Biol. 220, 381–399 (1991).
Kuriyan, J., Wilz, S., Karplus, M. & Petsko, G. A. J. molec. Biol. 192, 133–154 (1986).
Phillips, S. E. V. J. molec. Biol. 142, 531–554 (1980).
Tilton, R. F., Kuntz, I. D. & Petsko, G. A. Biochemistry 23, 2849–2857 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schlichting, I., Berendzen, J., Phillips, G. et al. Crystal structure of photolysed carbonmonoxy-myoglobin. Nature 371, 808–812 (1994). https://doi.org/10.1038/371808a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/371808a0
This article is cited by
-
Protein tertiary structure and the myoglobin phase diagram
Scientific Reports (2019)
-
Myoglobin ligand gate mechanism analysis by a novel 3D visualization technique
Journal of Mathematical Chemistry (2019)
-
Carbon monoxide binding properties of domain-swapped dimeric myoglobin
JBIC Journal of Biological Inorganic Chemistry (2015)
-
Assembly of nonheme Mn/Fe active sites in heterodinuclear metalloproteins
JBIC Journal of Biological Inorganic Chemistry (2014)
-
Time-resolved crystallography for protein structure: the case of heme proteins
Rendiconti Lincei (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.