Abstract
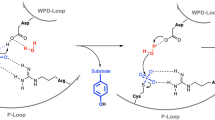
PROTEIN tyrosine phosphatases (PTPases) and kinases coregulate the critical levels of phosphorylation necessary for intracellular signalling, cell growth and differentiation1,2. Yersinia, the causative bacteria of the bubonic plague and other enteric diseases, secrete an active PTPase3, Yop51, that enters and suppresses host immune cells4,5. Though the catalytic domain is only ∼20% identical to human PTP1B6, the Yersinia PTPase contains all of the invariant residues present in eukaryotic PTPases7, including the nucleophilic Cys 403 which forms a phosphocysteine intermediate during catalysis3,8–10. We present here structures of the unliganded (2.5 Å resolution) and tungstate-bound (2.6 Å) crystal forms which reveal that Cys 403 is positioned at the centre of a distinctive phosphate-binding loop. This loop is at the hub of several hydrogen-bond arrays that not only stabilize a bound oxyanion, but may activate Cys 403 as a reactive thiolate. Binding of tungstate triggers a conformational change that traps the oxyanion and swings Asp 356, an important catalytic residue7, by ∼6 Å into the active site. The same anion-binding loop in PTPases is also found in the enzyme rhodanese11.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fischer, E. H., Charbonneau, H. & Tonks, N. K. Science 253, 401–406 (1991).
Walton, K. M. & Dixon, J. E. A. Rev. Biochem. 62, 101–120 (1993).
Guan, K. L. & Dixon, J. E. Science 249, 553–556 (1990).
Bolin, I. & Wolf-Watz, H. Molec. Microbiol. 2, 237–245 (1988).
Bliska, J. B., Guan, K. L., Dixon, J. E. & Falkow, S. Proc. natn. Acad. Sci. U.S.A. 88, 1187–1191 (1991).
Tonks, N. K., Diltz, C. D. & Fischer, E. H. J. biol. Chem. 263, 6722–6730 (1988).
Zhang, Z. Y., Wang, Y. & Dixon, J. E. Proc. natn. Acad. Sci. U.S.A. 91, 1624–1627 (1994).
Guan, K. L. & Dixon, J. E. J. biol. Chem. 266, 17026–17030 (1991).
Zhang, Z. Y. & Dixon, J. E. Biochemistry 32, 9340–9345 (1993).
Cho, H. et al. J. Am. chem. Soc. 114, 7296–7298 (1992).
Ploegman, J. H. et al. Nature 273, 124–129 (1978).
Barford, D., Flint, A. J. & Tonks, N. K. Science 263, 1397–1404 (1994).
Orengo, C. A. & Thornton, J. M. Structure 1, 105–120 (1993).
Zhang, Z. Y. & Dixon, J. E. Adv. Enzym. Relat. Areas molec. Biol. 68, 1–36 (1994).
Lewis, S. D., Johnson, F. A. & Shafer, J. A. Biochemistry 20, 48–51 (1981).
Pathak, D. & Ollis, D. J. molec. Biol. 214, 497–525 (1990).
Streuli, M., Krueger, N. X., Thai, T., Tang, M. & Saito, H. EMBO J. 9, 2399–2407 (1990).
Quiocho, F. A., Sack, J. S. & Vyas, N. K. Nature 329, 561–564 (1987).
Gregoret, L. M., Rader, S. D., Fletterick, R. J. & Cohen, F. E. Proteins 9, 99–107 (1991).
Åqvist, J., Luecke, H., Quiocho, F. A. & Warshel, A. Proc. natn. Acad. Sci. U.S.A. 88, 2026–2030 (1991).
Hol, W. G., van Duijnen, P. T. & Berendsen, H. J. Nature 273, 443–446 (1978).
Ploegman, J. H., Drent, G., Kalk, K. H. & Hol, W. G. J. molec. Biol. 127, 149–162 (1979).
Wierenga, R. K. Trans. Am. Crystallogr. Ass. 22, 49–62 (1986).
Zhang, Z. Y. et al. J. biol. Chem. 267, 23759–23766 (1992).
Howard, A. J., Nielsen, C. & Xuong, N. H. Meth. Enzym. 114, 452–472 (1985).
Furey, W. & Swaminathan, S. Am. Crystallogr. Ass. Meeting Abstr. 18, 73 (1990).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Steigemann, W. thesis, Technische Universität, Munich (1974).
Brünger, T. A. XPLOR Version 3.1 Manual (Yale Univ. Press, New Haven, 1993).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Zhang, Z. Y., Maclean, D., McNamara, D. J., Sawyer, T. K. & Dixon, J. E. Biochemistry 33, 2285–2290 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stuckey, J., Schubert, H., Fauman, E. et al. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 Å and the complex with tungstate. Nature 370, 571–575 (1994). https://doi.org/10.1038/370571a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/370571a0
This article is cited by
-
Tyrosine inhibits the Mycobacterium tuberculosis protein tyrosine phosphatase MptpA
Journal of Proteins and Proteomics (2023)
-
Computational identification of bioactive compounds from Cydonia oblonga Mill. against hepatocellular carcinoma by targeting pTEN and HBx-interacting protein
Journal of Molecular Modeling (2022)
-
Chemoselective synthesis and analysis of naturally occurring phosphorylated cysteine peptides
Nature Communications (2016)
-
Identification of candidate mimicry proteins involved in parasite-driven phenotypic changes
Parasites & Vectors (2015)
-
1H, 15N, and 13C backbone resonance assignments for the yersinia protein tyrosine phosphatase YopH
Biomolecular NMR Assignments (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.