Abstract
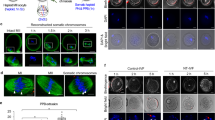
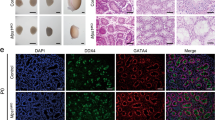
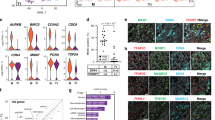
IN Xenopus the c-mos proto-oncogene product (Mos) is essential for the initiation of oocyte maturation1, for the progression from meiosis I to meiosis II2,3 and for the second meiotic metaphase arrest, acting as an essential component of the cytostatic factor CSF4,5. Its function in mouse oocytes is unclear6–9, however, as is the biological significance of c-mos mRNA expression in testes1,10 and several somatic tissues1,10,11. We have generated c-mos-deficient mice by gene targeting in embryonic stem cells. These mice grew at the same rate as their wild-type counterparts and reproduction was normal in the males, but the fertility of the females was very low. The c-mos-deficient female mice developed ovarian teratomas at a high frequency. Oocytes from these females matured to the second meiotic metaphase both in vivo and in vitro, but were activated without fertilization. The results indicate that in mice Mos plays a role in the second meiotic metaphase arrest, but does not seem to be essential for the initiation of oocyte maturation, spermatogenesis or somatic cell cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sagata, N., Oskarsson, M., Copeland, T., Brumbaugh, J. & Vande Woude, G. F. Nature 335, 519–525 (1988).
Kanki, J. P. & Donoghue, D. J. Proc. natn. Acad. Sci. U.S.A. 88, 5794–5798 (1991).
Daar, I., Paules, R. S. & Vande Woude, G. F. J. Cell Biol. 114, 329–335 (1991).
Masui, Y. & Markert, C. L. J. exp. Zool. 177, 129–146 (1971).
Sagata, N., Watanabe, N., Vande Woude, G. F. & Ikawa, Y. Nature 342, 512–518 (1989).
Zhao, X., Batten, B., Singh, B. & Arlinghaus, R. B. Oncogene 5, 1727–1730 (1990).
Zhao, X., Singh, B. & Batten, B. E. Oncogene 6, 43–49 (1991).
Paules, R. S., Buccione, R., Moschel, R. C., Vande Woude, G. F. & Eppig, J. J. Proc. natn. Acad. Sci. U.S.A. 86, 5395–5399 (1989).
O'Keefe, S. J., Wolfes, H., Kiessling, A. A. & Cooper, G. M. Proc. natn. Acad. Sci. U.S.A. 86, 7038–7042 (1989).
Propst, F. & Vande Woude, G. F. Nature 315, 516–518 (1985).
Propst, F., Rosenberg, M. P., Iyer, A., Kaul, K. & Vande Woude G. F. Molec. cell. Biol. 7, 1629–1637 (1987).
Yagi, T. et al. Analyt. Biochem. 214, 77–86 (1993).
Yagi, T. et al. Analyt. Biochem. 214, 70–76 (1993).
Herzog, N. K., Ramagli, L. S. & Arlinghaus, R. B. Oncogene 4, 1307–1315 (1989).
Hashimoto, N. & Kishimoto, T. Devl Biol. 126, 242–252 (1988).
Wolf, D. P. Devl Biol. 64, 1–10 (1978).
Inoue, M. & Wolf, D. P. Biol. Reprod. 13, 546–551 (1975).
Eppig, J. J., Kozak, L. P., Eicher, E. M. & Stevens, L. C. Nature 269, 517–518 (1977).
Furuno, N. et al. EMBO J. 13, 2399–2410 (1994).
Hanks, S. K., Quinn, A. M. & Hunter, T. Science 241, 42–52 (1988).
Hashimoto, N., Iwashita, S., Shoji-Kasai, Y., Kishimoto, T. & Imahori, K. Dev. Growth Differ. 32, 197–203 (1990).
Hogan, B., Constantini, F. & Lacy, E. Manipulating the Mouse Embryo 106 (Cold Spring Harbor Laboratory, New York, 1986).
Toyoda, Y., Yokoyama, M. & Hoshi, T. Jpn. J. Anim. Reprod. 16, 147–151 (1971).
Gratzner, H. G. Science 218, 474–475 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hashimoto, N., Watanabe, N., Furuta, Y. et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370, 68–71 (1994). https://doi.org/10.1038/370068a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/370068a0
This article is cited by
-
Recurrent spontaneous oocyte activation causes female infertility
Journal of Assisted Reproduction and Genetics (2022)
-
Meiotic Instability Generates a Pathological Condition in Mammalian Ovum
Stem Cell Reviews and Reports (2021)
-
A lack of coordination between sister-chromatids segregation and cytokinesis in the oocytes of B6.YTIR (XY) sex-reversed female mice
Scientific Reports (2017)
-
Aberrant Meiotic Prophase I Leads to Genic Male Sterility in the Novel TE5A Mutant of Brassica napus
Scientific Reports (2016)
-
Protein expression in human cumulus cells as an indicator of blastocyst formation and pregnancy success
Journal of Assisted Reproduction and Genetics (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.