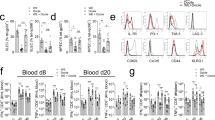
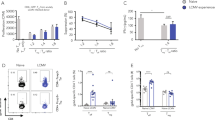
Abstract
VIRUSES that are non- or poorly cytopathic have developed various strategies to avoid elimination by the immune system and to persist in the host1–3. Acute infection of adult mice with the noncytopathic lymphocytic choriomeningitis virus (LCMV) normally induces a protective cytotoxic T-cell response that also causes immunopathology4–7. But some LCMV strains (such as DOCILE8 (LCMV-D) or Cl-13 Armstrong (Cl-13) 9) derived from virus carrier mice tend to persist after acute infection of adult mice without causing lethal immunopathological disease4,5. Tendency to persist correlates with tropism8,10, rapidity of virus spread8 and virus mutations11,12. We report here that these LCMV isolates may persist because they induce most of the specific antiviral CD8+ cytotoxic T cells so completely that they all disappear within a few days and therefore neither eliminate the virus nor cause lethal immunopathology. The results illustrate that partially and sequen-tially induced (protective) immunity or complete exhaustion of T-cell immunity (high zone tolerance) are quantitatively different points on the scale of immunity; some viruses exploit the latter possibility to persist in an immunocompetent host.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mims, C. A. The Pathogenesis of Infectious Disease (Academic, London, 1987).
Oldstone, M. B. A. Cell 56, 517–520 (1989).
Ahmed, R. & Stevens, J. G. in Virology (ed. Fields, B. N.) 241–265 (Raven, New York, 1990).
Hotchin, J. Monogr. Virol. 3, 1–211 (1971).
Lehmann-Grube, F. Virol. Monogr. 10, 1–173 (1971).
Cole, G. A., Nathanson, N. & Prendergast, R. A. Nature 238, 335–337 (1972).
Zinkernagel, R. M. & Doherty, P. C. Adv. Immun. 27, 52–142 (1979).
Pfau, C. J., Valenti, J. K., Pevear, D. C. & Hunt, K. D. J. exp. Med. 156, 79–89 (1982).
Ahmed, R., Salmi, A., Butler, L. D., Chiller, J. M. & Oldstone, M. B. A. J. exp. Med. 60, 521–540 (1984).
Ahmed, R. & Oldstone, M. B. A. J. exp. Med. 167, 1719–1724 (1988).
Ahmed, R. et al. J. Virol. 62, 3301–3308 (1988).
Salvato, M., Borrow, P., Shimomaye, E. & Oldstone, M. B. A. J. Virol. 65, 1863–1869 (1991).
Pircher, H. P., Bürki, K., Lang, R., Hengartner, H. & Zinkernagel, R. Nature 342, 559–561 (1989).
Marker, O. & Volkert, M. J. exp. Med 137, 1511–1525 (1973).
Cihak, J. & Lehmann-Grube, F. Immunology 34, 265 (1978).
Moskophidis, D., Assmann Wischer, U., Simon, M. M. & Lehmann Grube, F. Eur. J Immun. 17, 937–942 (1987).
Jamieson, B. D., Somasundaram, T. & Ahmed, R. J. Immun. 147, 3521–3529 (1991).
Schönrich, G. et al. Cell 65, 293–304 (1991).
Rocha, B. & von Boehmer, H. Science 251, 1225–1228 (1991).
Schwartz, R. H. Science 248, 1349–1356 (1990).
Herman, A., Kappler, J. W., Marrack, P. & Pullen, A. M. A. Rev. Immun. 9, 745–772 (1991).
Webb, S., Morris, C. & Sprent, J. Cell 63, 1249–1256 (1990).
Rammensee, H. G., Kroschewski, R. & Frangoulis, B. Nature 339, 541–544 (1989).
Ignatowicz, L., Kappler, J. & Marrack, P. J. exp. Med. 175, 917–923 (1992).
Saron, M. F., Shidani, B., Nahori, M. A., Guillon, J. C. & Truffa Bachi, P. J. Virol. 64, 4076–4083 (1990).
Campbell, I. L., Lepay, D. A. & Oldstone, M. B. A. J. Cell. Biochem. 16, 141 (1992).
Jacobs, R. P. & Cole, G. A. J. Immun. 117, 1004–1009 (1976).
Odermatt, B., Eppler, M., Leist, T. P., Hengartner, H. & Zinkernagel, R. M. Proc. natn. Acad. Sci. U.S.A. 88, 8252–8256 (1991).
Borrow, P., Tishon, A. & Oldstone, M. B. A. J. exp. Med. 174, 203–212 (1991).
Tishon, A., Southern, P. J. & Oldstone, M. B. A. J. Immun. 140, 1280–1284 (1988).
Zinkernagel, R. M., Leist, T. P., Hengartner, H. & Althage, A. J. exp. Med. 162, 2125–2141 (1985).
Doherty, P. C., Zinkernagel, R. M. & Ramshaw, I. A. J. Immun. 112, 1548–1552 (1974).
Dunlop, M. B. C. & Blanden, R. V. J. exp. Med 145, 1131–1143 (1977).
Gilden, D. H., Cole, G. A., Monjan, A. A. & Nathanson, N. J. exp. Med. 135, 860–873 (1972).
Stitz, L. Eur. J. Immun. 22, 1995–2001 (1992).
Peters, M. et al. Hepatology 13, 977–994 (1991).
Clerici, M. et al. J. Immun. 146, 2214–2219 (1991).
Groux, H. et al. J. exp. Med. 175, 331–340 (1992).
Miller, R. G. Nature 287, 544 (1980).
Mitchison, N. A. Proc. R Soc. 161, 275–292 (1964).
Pircher, H. P. et al. Nature 346, 629–633 (1990).
Pircher, H. P. et al. Eur. I Immun. 22, 399–404 (1992).
Tomonari, K. Immunogenetics 28, 455–458 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moskophidis, D., Lechner, F., Pircher, H. et al. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993). https://doi.org/10.1038/362758a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/362758a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.