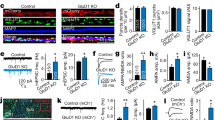
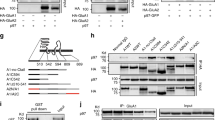
Abstract
THE major postsynaptic density (PSD) protein1,2 at glutaminergic synapses is calcium/calmodulin-dependent protein kinase II (CaM-K II), but its function in the PSD is not known. We have examined glutamate receptors (GluRs) as substrates for CaM-K II because (1) they are colocalized in the PSD3, (2) cloned GluRs4–7 contain consensus phosphorylation sites for protein kinases including CaM-K II, and (3) several GluRs are regulated by other protein kinases8–12. Regulation of GluRs, which are involved in excitatory synaptic transmission and in mechanisms of learning and memory13, by CaM-K II is of interest because of the postulated role of CaM-K II in synaptic plasticity14,15 and its known involvement in induction of long-term potentiation16. Furthermore, mice lacking the major neural isoform of CaM-K II exhibit deficits in models of learning and memory that require hippocampal input17,18. We report here that CaM-K II phosphorylates GluR in several in vitro systems, including the PSD, and that activated CaM-K II enhances kainate-induced ion current three- to fourfold in cultured hippocampal neurons. These results are consistent with a role for PSD CaM-K II in strengthening postsynaptic GluR responses in synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kennedy, M. B., Bennett, M. K. & Erondu, N. G. Proc. natn. Acad. Sci. U.S.A. 80, 7357–7361 (1983).
Kelly, P. T., McGuiness, T. L. & Greengard, P. Proc. natn. Acad Sci. U.S.A. 81, 945–949 (1984).
Wu, K., Carlin, R. & Siekevitz, P. J. Neurochemistry 46, 831–841 (1986).
Boulter, J. et al. Science 249, 1033–1037 (1990).
Keinänen, K. et al. Science 249, 556–560 (1990).
Egebjerg, J., Bettler, B., Hermans-Borgmeyer, I. & Heinemann, S. Nature 351, 745–748 (1991).
Moriyoshi, K. et al. Nature 354, 31–37 (1991).
Knapp, A. G., Schmidt, K. F. & Dowling, J. E. Proc. natn. Acad. Sci. U.S.A. 87, 767–771 (1990).
Wang, L.-Y., Salter, M. W. & MacDonald, J. F. Science 253, 1132–1135 (1991).
Greengard, P., Jen, J., Nairn, A. C. & Stevens, C. F. Science 253, 1135–1138 (1991).
Keller, B. U., Hollmann, M., Heinemann, S., & Konnerth, A. EMBO J. 11, 891–896 (1992).
Wang, L. Y., Dudek, E. M., Browning, M. & MacDonald, J. F. Neurosci. Abstr. 18, 653 (1992).
Westbrook, G. L. & Jahr, C. E. Semin. Neurosci. 1, 103–114 (1989).
Lisman, J. E. & Goldring, M. A. Proc. natn. Acad. Sci. U.S.A. 85, 5320–5324 (1988).
Miller, S. G. & Kennedy, M. B. Cell 44, 861–870 (1986).
Malinow, R., Schulman, H. & Tsien, R. W. Science 245, 862–866 (1989).
Silva, A. J. et al. Science 257, 201–206 (1992).
Silva, A. J., et al. Science 257, 206–210 (1992).
Siekevitz, P. Proc. natn. Acad. Sci. U.S.A. 82, 3494–3498 (1985).
Raymond, L. A., Blackstone, C. D. & Huganir, R. L. Nature 361, 637–641 (1993).
Fukunaga, K. & Soderling, T. R. Molec. cell. Neurosci. 1, 133–138 (1990).
MacKintosh, C. et al. FEBS Lett. 264, 187–193 (1990).
Wenthold, R. J., Yokotani, N., Doi, K. & Wada, K., J. biol. Chem. 267, 501–507 (1992).
Manabe, T., Renner, P. & Nicoll, R. A. Nature 355, 50–55 (1992).
Summners, M. D. & Smith, G. E. Texas Agricultural Experiment Station Bulletin No. 1555 (1988).
Fukunaga, K., Soderling, T. R. & Miyamoto, E. J. biol. Chem. 267, 22527–22533 (1992).
Brickey, D. A., Colbran, R. J., Fong, Y. L. & Soderling, T. R. Biochem. biophys. Res. Commun. 173, 578–584 (1990).
Rich, D. P., Colbran, R. J., Schworer, C. M. & Soderling, T. R. J. Neurochem. 53, 807–816 (1989).
Smith, M. K., Colbran, R. J. & Soderling, T. R., J. biol. Chem. 265, 1837–1840 (1990).
Lester, R. A. J. et al. Molec. Pharmac. 35, 565–570 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McGlade-McCulloh, E., Yamamoto, H., Tan, SE. et al. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362, 640–642 (1993). https://doi.org/10.1038/362640a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/362640a0
This article is cited by
-
PKA drives an increase in AMPA receptor unitary conductance during LTP in the hippocampus
Nature Communications (2021)
-
Neurons promote encephalitogenic CD4+ lymphocyte infiltration in experimental autoimmune encephalomyelitis
Scientific Reports (2020)
-
Reduced CaM Kinase II and CaM Kinase IV Activities Underlie Cognitive Deficits in NCKX2 Heterozygous Mice
Molecular Neurobiology (2017)
-
siRNA capsulated brain-targeted nanoparticles specifically knock down OATP2B1 in mice: a mechanism for acute morphine tolerance suppression
Scientific Reports (2016)
-
Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.