Abstract
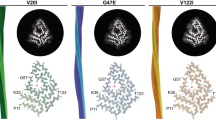
HEREDITARY non-neuropathic systemic amyloidosis (Ostertag-type)1 is a rare autosomal dominant disease in which amyloid deposition in the viscera is usually fatal by the fifth decade. In some families it is caused by mutations in the apolipoprotein AI gene2,3 but in two unrelated English families under our care the amyloid deposits did not contain apoAI, despite a report that this may have been the case in one of them4. Lysozyme is a ubiquitous bacteriolytic enzyme present in external secretions5 and in poly-morphs and macrophages, but its physiological role is not always clear6. Here we report that in these two families, lysozyme is the amyloid fibril protein. Affected individuals are heterozygous for point mutations in the lysozyme gene that cause substitution of highly conserved residues, namely threonine for isoleucine at position 56 in one family, and histidine for aspartic acid at residue 67 in the other. Amyloid fibrils from one individual were composed of the full-length Thr-56 variant lysozyme molecule. To our knowledge, this is the first report of naturally occurring variants of human lysozyme and of lysozyme-associated disease. As the structures of human7 and hen egg-white lysozyme8 are known to atomic resolution and their folding and structure–function relationships have been exhaustively analysed, our observations should provide a powerful model for understanding amyloidogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ostertag, B. Zentralbl. Aug. Path. 56, 253–254 (1932).
Jones, L. A., Harding, J. A., Cohen, A. S. & Skinner, M. in Amyloid and Amyloidosis 1990 (eds Natvig, J. B. et al.) 385–388 (Kluwer Academic, Dordrecht, 1991).
Soutar, A. K. et al. Proc. natn. Acad. Sci. U.S.A. 89, 7389–7393 (1992).
Zalin, A. M., Jones, S., Fitch, N. J. S. & Ramsden, D. B. Q. Jl Med. 81, 945–956 (1991).
Fleming, A. Roy. Soc. Proc. B93, 306–317 (1922).
Jollès, P. & Jollès, J. Molec. cell. Biochem. 63, 165–189 (1984).
Artymiuk, P. J. & Blake, C. C. F. J. molec. Biol. 152, 737–762 (1981).
Blake, C. C. F. et al. Nature 206, 757–761 (1965).
Athanasou, N. A. & Sallie, B. Histopathology 20, 41–46 (1992).
Patrinely, J. R. & Koch, D. D. Arch. Ophthalmol. 110, 882–885 (1992).
Imoto, T., Johnson, L., North, A., Phillips, D. & Rupley, J. in The Enzymes (ed. Boyer, P. D.) 665–868 (Academic, New York, 1972).
Sidman, K., George, D., Barkers, W. & Hunt, L. Nucleic Acids Res. 16, 869–781 (1988).
Rupley, J. et al. in Lysozyme (eds Osserman, E. F., Canfield, R. E. & Beychok, S.) 251–267 (Academic, New York, 1974).
Artymiuk, P. J. et al. Nature 280, 563–586 (1979).
Wilson, K., Malcolm, B. & Matthews, B. J. biol. Chem. 267, 10842–10849 (1992).
Brooks, B. et al. J. comp. Chem. 4, 187–217 (1983).
Alber, T., Dao-pin, S., Nye, J. A., Muchmore, D. C. & Matthews, B. W. Biochemistry 26, 3754–3758 (1987).
Shirley, B. A., Stanssens, P., Hahn, U. & Pace, C. N. Biochemistry 31, 725–732 (1992).
Pepys, M. B. in Immunological Diseases 4th edn Vol. 1 (eds Samter, M., Talmage, D. W., Frank, M. M., Austen, K. F. & Claman, H. N.) 631–674 (Little Brown, Boston, 1988).
Lanham, J. G., Meltzer, M. L., de Beer, F. C., Hughes, G. R. V. & Pepys, M. B. Q. Jl Med. 51, 25–32 (1982).
Hawkins, P. N., Lavender, J. P. & Pepys, M. B. New Engl. J. Med 323, 508–513 (1990).
Puchtler, H., Sweat, F. & Levine, M. J. Histochem. Cytochem, 10, 355–364 (1962).
Pras, M., Schubert, M., Zucker-Franklin, D., Rimon, A. & Franklin, E. C. J. clin. Invest. 47, 924–933 (1968).
Sternberger, L. A. Immunocytochemistry 2nd edn (Wiley, New York, 1979).
Totty, N. F., Waterfield, M. D. & Hsuan, J. J. Protein Sci. 1, 1215–1224 (1992).
Hsuan, J. J., Totty, N. & Waterfield, M. D. Biochem. J. 262, 659–663 (1989).
Yap, K. W. & Thompson, R. C. A. Parasit. Today 3, 220–222 (1987).
Talmud, P. et al. Atherosclerosis 89, 137–141 (1991).
Peters, C. W. B., Kruse, U., Pollwein, R., Grzeschik, K.-H. & Sippel, A. E. Eur. J. Biochem. 182, 507–516 (1989).
Saiki, R. K. et al. Science 239, 487–491 (1988).
Casanova, J.-L., Pannetier, C., Jaulin, C. & Kourilsky, P. Nucleic Acids Res. 18, 4028 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pepys, M., Hawkins, P., Booth, D. et al. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature 362, 553–557 (1993). https://doi.org/10.1038/362553a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/362553a0
This article is cited by
-
Renal amyloidosis: an update on diagnosis and pathogenesis
Protoplasma (2020)
-
Systemic Amyloidosis: a Contemporary Overview
Clinical Reviews in Allergy & Immunology (2020)
-
Typing of hereditary renal amyloidosis presenting with isolated glomerular amyloid deposition
BMC Nephrology (2019)
-
Hereditary renal amyloidosis with a variant lysozyme p.Trp82Arg in a Chinese family: case report and literature review
BMC Nephrology (2019)
-
Kinetic Control of Parallel versus Antiparallel Amyloid Aggregation via Shape of the Growing Aggregate
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.