Abstract
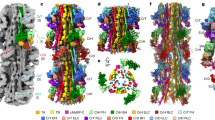
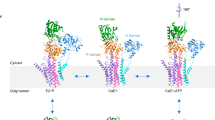
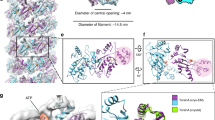
THE ATP-driven calcium pump (Ca2+-ATPase) is an integral membrane protein (Mr 11 OK) which relaxes striated muscle by pumping calcium out of the cytoplasm into the sarcoplasmic reticulum against a large concentration gradient1. Recent efforts have attempted to relate the sequence of Ca2+-ATPase to its structure and function. In particular, site-directed mutagenesis has identified critical amino-acid residues2–6, and its predicted secondary structure, which includes ten transmembrane helices7, has gained experimental support8–10. But direct visualization of the molecule has so far been limited to the cytoplasmic domains at low resolution11, 12. We present here the three-dimensional structure of Ca2+-ATPase in the native sarcoplasmic reticulum membrane at 14 Å resolution, determined by cryo-electron microscopy and helical image analysis. The structure shows an unexpected transmembrane organization, consisting of three distinct segments, one of which is highly inclined. These features can be related to earlier predictions of secondary structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Inesi, G., Lewis, D., Nikic, D., Hussain, A. & Kirtley, M. E. Adv. Enzym. 65, 185–215 (1992).
Clarke, D. M., Loo, T. W. & MacLennan, D. H. J. biol. Chem. 265, 22223–22227 (1990).
Clarke, D. M., Loo, T. W. & MacLennan, D. H. J. biol. Chem. 265, 6262–6267 (1990).
Clarke, D. M. et al. J. biol. Chem. 264, 11246–11251 (1989).
Vilsen, B., Andersen, J. P., Clarke, D. M. & MacLennan, D. H. J. biol. Chem. 264, 21024–21030 (1989).
Andersen, J. P., Vilsen, B., Leberer, E. & MacLennan, D. H. J. biol. Chem. 264, 21018–21023 (1989).
MacLennan, D. H., Brandl, C. J., Korczak, B. & Green, N. M. Nature 316, 696–700 (1985).
Matthews, I., Sharma, R. P., Lee, A. G. & East, J. M. J. biol. Chem. 265, 18737–18740 (1990).
Clarke, D. M., Loo, T. W. & MacLennan, D. H. J. biol. Chem. 265, 17405–17408 (1990).
Sachs, G. et al. J. Bioenerg. Biomembr. 24, 301–308 (1992).
Taylor, K. A., Dux, L. & Martonosi, A. J. molec. Biol. 187, 417–427 (1986).
Castellani, L., Hardwlcke, P. M. & Vibert, P. J. molec. Biol. 185, 579–594 (1985).
Dux, L. & Martonosi, A. J. biol. Chem. 258, 2599–2603 (1983).
Toyoshima, C. & Unwin, N. J. Cell Biol. 111, 2623–2635 (1990).
Herbette, L. et al. Biochim. biophys. Acta 817, 103–122 (1985).
Dupont, Y., Harrison, S. C. & Hasselbach, W. nature 244, 555–558 (1973).
Brandl, C. J., Green, N. M., Korczak, B. & MacLennan, D. H. Cell 44, 597–607 (1986).
Green, N. M. Biochem. Soc. Trans. 17, 970–972 (1989).
Clarke, D. M., Loo, T. W., Inesi, G. & MacLennan, D. H. Nature 339, 476–478 (1989).
Green, N. M. & Stokes, D. L. Acta Physiol. scand. 146, 59–68 (1992).
Meissner, G., Conner, G. E. & Fleischer, S. Biochim. biophys. Acta 298, 246–269 (1973).
Toyoshima, C. Ultramicroscopy 30, 439–444 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toyoshima, C., Sasabe, H. & Stokes, D. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature 362, 469–471 (1993). https://doi.org/10.1038/362469a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/362469a0
This article is cited by
-
Targeting oncogenic Notch signaling with SERCA inhibitors
Journal of Hematology & Oncology (2021)
-
ATP4A gene regulatory network for fine-tuning of proton pump and ion channels
Systems and Synthetic Biology (2013)
-
Crystal structure of a copper-transporting PIB-type ATPase
Nature (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.