Abstract
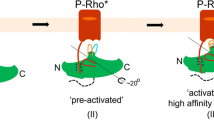
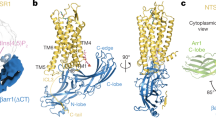
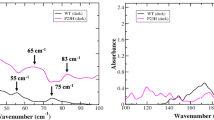
Retinal arrestin is the essential protein for the termination of the light response in vertebrate rod outer segments. It plays an important role in quenching the light-induced enzyme cascade by its ability to bind to phosphorylated light-activated rhodopsin (P-Rh*). Arrestins are found in various G-protein-coupled amplification cascades. Here we report on the three-dimensional structure of bovine arrestin (relative molecular mass, 45,300) at 3.3 Å resolution. The crystal structure comprises two domains of antiparallel β-sheets connected through a hinge region and one short α-helix on the back of the amino-terminal fold. The binding region for phosphorylated light-activated rhodopsin is located at the N-terminal domain, as indicated by the docking of the photoreceptor to the three-dimensional structure of arrestin. This agrees with the interpretation of binding studies on partially digested and mutated arrestin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kühn, H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry 17, 4289–4395 (1978).
Fung, B. K.-K. & Stryer, L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc. Natl Acad. Sci. USA 77, 2500–2504 (1980).
Fung, B. K.-K., Hurley, J. B. & Stryer, L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc. Natl Acad. Sci. USA 78, 152–156 (1981).
Uchida, S., Wheeler, G. L., Yamazaki, A. & Bitensky, M. W. AGTP-protein activator of phosphodiesterase which forms in response to bleached rhodopsin. J. Cyclic Nucl. Res. 7, 95–104 (1981).
Vuong, T. M., Chabre, M. & Stryer, L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature 311, 659–661 (1984).
Wilden, U. & Kühn, H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry 21, 3014–3022 (1982).
Miller, J. L., Fox, D. A. & Litman, B. J. Amplification of phosphodiesterase activation is greatly reduced by rhodopsin phosphorylation. Biochemistry 25, 4983–4988 (1986).
Wilden, U., Hall, S. W. & Kühn, H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc. Natl Acad. Sci. USA 83, 1174–1178 (1986).
Wilden, U. Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry 34, 1446–1454 (1995).
Schleicher, A., Kühn, H. & Hofmann, K. P. Kinetics, binding constant, and activation energy of the 48-kDa protein-rhodopsin complex by extra-metarhodopsin II. Biochemistry 28, 1770–1775 (1989).
Wilden, U., Wüst, E., Weyand, I. & Kühn, H. Rapid affinity purification of retinal arrestin (48kDa protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett. 207, 292–295 (1986).
Kleywegt, G. J. Making the most of your search model. CCP4/ESF-EACBM News. Prot. Crystallogr. 32, 32–36 (1996).
Brunger, A. T. X-PLOR, Version 3.1-3.851, A System for X-ray Crystallography and NMR (Yale University, New Haven, CT, 1992–1997).
Shinohara, T.et al. Primary and secondary structure of bovine retinal S antigen (48-kDa). Proc. Natl Acad. Sci. USA 84, 6974–6979 (1987).
Palczewski, K., Pulvermüller, A., Buczylko, J. & Hofmann, K. P. Phosphorylated rhodopsin and heparin induce similar conformational changes in arrestin. J. Biol. Chem. 266, 18649–18654 (1991).
Gurevich, V. V. & Benovic, J. L. Visual arrestin interaction with rhodopsin. J. Biol. Chem. 268, 11628–11638 (1993).
Gurevich, V. V. & Benovic, J. L. Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into high affinity binding state. Mol. Pharmacol. 51, 161–169 (1997).
Attwood, T. K.et al. Novel developments with the PRINTS protein fingerprint database. Nucleic Acids Res. 25, 212–216 (1997).
Xinyu, Z., Palczewski, K. & Ohguro, H. Mechanism of rhodopsin phosphorylation. Biophys. Chem. 56, 183–188 (1995).
Holm, L. & Sander, C. The FSSP database of structurally aligned protein fold families. Nucleic Acids Res. 22, 3600–3609 (1994).
Noel, J. P., Hamm, H. E. & Sigler, P. B. The 2.2 Å crystal structure of transducin-α complexed with GTPγS. Nature 366, 654–366 (1993).
Lohse, M. J., Benovic, J. L., Codina, J., Caron, M. G. & Lefkowitz, R. J. β-Arrestin: a protein that regulates β-adrenergic receptor function. Science 248, 1547–1550 (1990).
Pogozheva, I. D., Lomize, A. L. & Mosberg, H. I. The transmembrane 7-α-bundle of rhodopsin: distance geometry calculation with hydrogen bonding constraints. Biophys. J. 70, 1963–1985 (1997).
Wilden, U., Choe, H.-W., Krafft, B. & Granzin, J. Crystallization and preliminary X-ray analysis of arrestin from bovine rod outer segment. FEBS Lett. 415, 268–270 (1997).
Collaborative Computational Project, Number4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D50, 760–763 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A47, 110–119 (1991).
Evans, S. V. SETOR: hardware lighted three-dimensional solid model representation of macromolecules. J. Mol. Graphics 11, 134–138 (1993).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: patter recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Acknowledgements
We thank A. Cousin and R. Esser for the arrestin protein preparation from bovine eyes; L. A. Donoso for the arrestin antibodies; U. B. Kaupp for continuous support of the project; K.-P. Hofmann and M. J. Lohse for helpful discussions. This work was funded by a grant from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Granzin, J., Wilden, U., Choe, HW. et al. X-ray crystal structure of arrestin from bovine rod outer segments. Nature 391, 918–921 (1998). https://doi.org/10.1038/36147
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/36147
This article is cited by
-
Dynamic Nature of Proteins is Critically Important for Their Function: GPCRs and Signal Transducers
Applied Magnetic Resonance (2024)
-
Functional compartmentalization of photoreceptor neurons
Pflügers Archiv - European Journal of Physiology (2021)
-
Phosphorylated peptide of G protein-coupled receptor induces dimerization in activated arrestin
Scientific Reports (2020)
-
Many faces of the GPCR-arrestin interaction
Archives of Pharmacal Research (2020)
-
Plethora of functions packed into 45 kDa arrestins: biological implications and possible therapeutic strategies
Cellular and Molecular Life Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.