Abstract
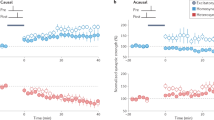
Information is stored in neural circuits through long-lasting changes in synaptic strengths1,2. Most studies of information storage have focused on mechanisms such as long-term potentiation and depression (LTP and LTD), in which synaptic strengths change in a synapse-specific manner3,4. In contrast, little attention has been paid to mechanisms that regulate the total synaptic strength of a neuron. Here we describe a new form of synaptic plasticity that increases or decreases the strength of all of a neuron's synaptic inputs as a function of activity. Chronic blockade of cortical culture activity increased the amplitude of miniature excitatory postsynaptic currents (mEPSCs) without changing their kinetics. Conversely, blocking GABA (γ-aminutyric acid)-mediated inhibition initially raised firing rates, but over a 48-hour period mESPC amplitudes decreased and firing rates returned to close to control values. These changes were at least partly due to postsynaptic alterations in the response to glutamate, and apparently affected each synapse in proportion to its initial strength. Such ‘synaptic scaling’ may help to ensure that firing rates do not become saturated during developmental changes in the number and strength of synaptic inputs5, as well as stabilizing synaptic strengths during Hebbian modification6,7 and facilitating competition between synapses7,8,9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hebb, D. O. The Organization of Behavior: a Neurophysiological Theory (Wiley, New York, 1949).
Stent, G. S. Aphysiological mechanism for Hebb's postulate of learning. Proc. Natl Acad. Sci. USA 70, 997–1001 (1973).
Madison, D. V.et al. Mechanisms underlying long-term potentiation of synaptic transmission. Annu. Rev. Neurosci. 14, 379–397 (1991).
Linden, D. J. & Connor, J. A. Long-term synaptic depression. Annu. Rev. Neurosci. 18, 319–357 (1995).
Shatz, C. J. Impulse activity and the patterning of connections during CNS development. Neuron 5, 745–756 (1990).
Bienenstock, E. L.et al. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48 (1982).
Miller, K. D. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron 17, 371–374 (1996).
Lo, Y. J. & Poo, M. Activity-dependent synaptic competition in vitro: heterosynaptic suppression of developing synapses. Science 254, 1019–1022 (1991).
Colman, H.et al. Alterations in synaptic strength preceding axon withdrawal. Science 275, 356–361 (1997).
Bekkers, J. M.et al. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc. Natl Acad. Sci. USA 87, 5359–5362 (1990).
Hestrin, S. Activation and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron 9, 991–999 (1992).
Kirkwood, A. & Bear, M. F. Hebbian synapses in visual cortex. J. Neurosci. 14, 1634–1645 (1994).
Manabe, T.et al. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature 355, 50–55 (1992).
Malgaroli, A.et al. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature 357, 134–139 (1992).
Wyllie, D. J.et al. Arise in postsynaptic calcium potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron 12, 127–138 (1994).
Micheva, K. D. & Beaulieu, C. An anatomical substrate for experience-dependent plasticity of the rat barel field cortex. Proc. Natl Acad. Sci. USA 92, 11834–11838 (1995).
Benson, D. L. & Cohen, P. A. Activity-independent segregation of excitatory and inhibitory synaptic terminals in cultured hippocampal neurons. J. Neurosci. 16, 6424–6432 (1996).
Rutherford, L. C.et al. BDNF mediates the activity-dependent regulation of inhibition in neocortical cultures. J. Neurosci. 17, 4527–4535 (1997).
Ramakers, G. J. A.et al. Development in the absence of spontaneous bioelectric activity results in increased stereotyped burst firing in cultures of dissociated cerebral cortex. Exp. Brain Res. 79, 157–166 (1990).
Axelsson, J. & Thesleff, S. Astudy of supersensitivity in denervated mammalian skeletal muscle. J.Physiol. (Lond.) 149, 178–193 (1957).
Berg, D. K. & Hall, Z. W. Increased extrajunctional acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J. Physiol (Lond.) 244, 659–676 (1975).
McGlade-McCulloh, E.et al. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362, 640–642 (1993).
Barria, A.et al. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276, 2042–2045 (1997).
Gerfin-Moser, A.et al. Alterations in glutamate but not GABAA receptor subunit expression as a consequence of epileptiform activity in vitro. Neuroscience 67, 849–865 (1995).
Liang, F. & Jones, E. G. Differential and time-dependent changes in gene expression for type II calcium/calmodulin-dependent protein kinase, 67 kDa glutamic acid decarboxylase, and glutamate receptor subunits in tetanus toxin-induced focal epilepsy. J. Neurosci. 17, 2168–2180 (1997).
Liao, D.et al. Activation of postysnaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375, 400–404 (1995).
Wu, G.-Y.et al. Maturation of a central glutamatergic synapse. Science 274, 972–976 (1996).
Blue, M. E. & Parnavelas, J. G. The formation and maturation of synapses inthe visual cortex of the rat. II. Quantitative analysis. J. Neurocytol. 12, 697–712 (1983).
Liu, G. & Tsien, R. W. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature 375, 404–408 (1995).
Miller, K. D. & MacKay, D. J. C. The role of constraints in Hebbian learning. Neur. Comput. 6, 100–126 (1993).
Acknowledgements
We thank H. Lauer for technical assistance, and L. F. Abbott, J. Lisman, E. Marder and M. Mauk for discussions and advice. This work was supported by the Whitehall Foundation, NSF, NIH and the Sloan Foundation. K.R.L. was supported by an HHMI predoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turrigiano, G., Leslie, K., Desai, N. et al. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 (1998). https://doi.org/10.1038/36103
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/36103
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.