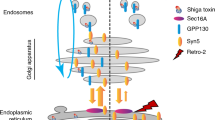
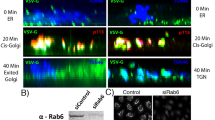
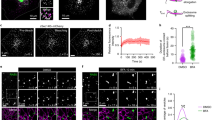
Abstract
SHIGA toxin and some other protein toxins that act on targets in the cytosol have previously been shown to enter the trans-Golgi network1–9. Transport by this route may be necessary for translocation of the toxin to the cytosol and for intoxication5–9, but it is not known whether the enzymatically active part of the toxins actually enters the cytosol from the fraiw-Golgi network. It has been suggested that such toxins are transported in a retrograde manner to the endoplasmic reticulum and that translocation occurs in this organelle10, but retrograde transport of endocytosed material beyond the trans-Golgi network has never been demonstrated. Here we show that in butyric acid-treated A431 cells endocytosed Shiga toxin is not only transported to the trans-Golgi network, but also to all Golgi stacks, to the endoplasmic reticulum and to the nuclear envelope. Furthermore, butyric acid sensitizes the cells to Shiga toxin, which is consistent with the possibility that retrograde transport is required for translocation of the toxin to the cytosol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
van Deurs, B. et al. J. Cell Biol. 106, 253–267 (1988).
Hansen, S. H., Petersen, O. W., Sandvig, K., Olsnes, S. & van Deurs, B. Expl Cell Res. 185, 373–386 (1989).
Sandvig, K., Olsnes, S., Brown, J. E., Petersen, O. W. & van Deurs, B. J. Cell Biol. 108, 1331–1343 (1989).
Sandvig, K., Prydz, K., Ryd, M. & van Deurs, B. J. Cell Biol. 113, 553–562 (1991).
van Deurs, B., Petersen, O. W., Olsnes, S. & Sandvig, K. Int. Rev. Cyt. 117, 131–177 (1989).
Sandvig, K., Tønnessen, T. I. & Olsnes, S. Cancer Res. 46, 6418–6422 (1986).
van Deurs, B., Tønnessen, T. I., Petersen, O. W., Sandvig, K. & Olsnes, S. J. Cell Biol. 102, 37–47 (1986).
Youle, R. J. & Colombatti, M. J. biol. Chem. 262, 4676–4682 (1987).
Olsnes, S. & Sandvig, K. in Immunotoxins (ed. Frankel, A. E.) Vol. 1 39–73 (Kluwer Academic, Boston, 1988).
Pelham, H. R. B., Roberts, L. M. & Lord, J. M. Trends Cell Biol. 2, 183–185 (1992).
Kartenbeck, J., Stukenbrok, H. & Helenius, A. J. Cell Biol. 109, 2721–2729 (1989).
Lewis, M. J. & Pelham, R. B. Cell 68, 353–364 (1992).
Van, P. N., Peter, F. & Söling, H.-D. J. biol. Chem. 264, 17494–17501 (1989).
Seetharam, S., Chaudhary, V. K., Fitzgerald, D. & Pastan, I. J. biol. Chem. 266, 17376–17381 (1991).
Chaudhary, V. K., Jinno, Y., Fitzgerald, D. & Pastan, I. Proc. natn. Acad. Sci. U.S.A. 87, 308–312 (1990).
Kozlov, Y. V., Kabishev, A. A., Lukyanov, E. V. & Bayev, A. A. Gene 67, 213–221 (1988).
Strockbine, N. A., Jackson, M. P., Sung, L. M., Holmes, R. K. & O'Brien, A. J. Bact. 170, 1116–1122 (1988).
Seidah, N. G. et al. J. biol. Chem. 261, 13928–13931 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sandvig, K., Garred, Ø., Prydz, K. et al. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 358, 510–512 (1992). https://doi.org/10.1038/358510a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358510a0
This article is cited by
-
Eliglustat prevents Shiga toxin 2 cytotoxic effects in human renal tubular epithelial cells
Pediatric Research (2022)
-
RAB5A and TRAPPC6B are novel targets for Shiga toxin 2a inactivation in kidney epithelial cells
Scientific Reports (2020)
-
A mosquito salivary protein promotes flavivirus transmission by activation of autophagy
Nature Communications (2020)
-
Functional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2
Nature Chemical Biology (2020)
-
The role of PS 18:0/18:1 in membrane function
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.