Abstract
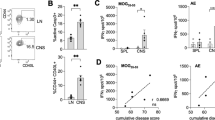
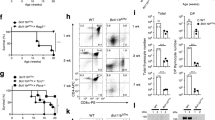
IMMUNIZATION with myelin basic protein (MBP) induces experimental allergic encephalomyelitis (EAE), a prototype of CD4+ T-cell mediated autoimmune disease. In rodents, MBP-reactive T-cell clones are specific for a single, dominant determinant on MBP and use a highly restricted number of T-cell receptor genes1–3. Accordingly, EAE has been prevented by various receptor-specific treatments1–8, suggesting similar strategies may be useful for therapy of human autoimmune disease. Here we report that in (SJL x B 10.PL)F1 mice, immune dominance of a single determinant, MBP: Ac1–11, is confined to the inductive phase of EAE. In mice with chronic EAE, several additional determinants of MBP in peptides 35–47, 81–100 and 121–140 recall proliferative responses. Most importantly, reactivity to the latter determinants was also detected after induction of EAE with MBP peptide Ac1–11 alone; this demonstrates priming by endogenous MBP determinants. Thus, determinants of MBP that are cryptic9–11 after primary immunization can become immunogenic in the course of EAE. Diversification of the autoreactive T-cell repertoire due to 'determinant spreading' has major implications for the pathogenesis of, and the therapeutic approach to, T-cell driven autoimmune disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Urban, J. L. et al. Cell 54, 577–592 (1988).
Acha-Orbea, H. et al. Cell 54, 263–273 (1988).
Owhashi, M. & Heber-Katz, E. J. exp. Med. 168, 2153–2164 (1988).
Ben-Nun, A., Wekerle, H. & Cohen, I. R. Nature 292, 60–61 (1981).
Vandenbark, A. A., Hashim, G. & Offner, H. Nature 341, 541–544 (1989).
Howell, M. D. et al. Science 246, 668–670 (1989).
Offner, H., Hashim, G. A. & Vandenbark, A. Science 251, 430–432 (1991).
Zaller, D. M., Osman, G. Kanagawa, O. & Hood, L. J. exp. Med. 171, 1943–1955 (1990).
Gammon, G. & Sercarz, E. E. Nature 342, 183–185 (1989).
Benichou, G. et al. J. exp. Med. 172, 1341–1346 (1990).
Gammon, G., Sercarz, E. E. & Benichou, G. Immun. Today 12, 193–195 (1991).
Linthicum, D. S. & Frelinger, J. A. J. exp. Med. 155, 31–40 (1982).
Sakai, K. et al. J. Neuroimmun. 19, 21–32 (1988).
Hood, L. et al. Cold Spring Harb. Symp. quant. molec. Biol. 54, 859–874 (1989).
Zamvil, S. S. et al. J. exp. Med. 168, 1181–1186 (1988).
Perry, L. L. & Barzaga, M. E. J. Immun. 138, 1434–1441 (1987).
Perry, L. L., Barzaga-Gilbert, E. & Trotter, J. L. J. Neuroimmun. 33, 7–15 (1991).
McCarron, R. M., Fallis, R. J. & McFarlin, D. E. J. Neuroimmun. 29, 73–79 (1990).
Sarvetnick, N. Nature 346, 844–847 (1990).
Heber-Katz, E. & Acha-Orbea, H. Immun. Today 10, 164–169 (1989).
Fritz, R. B. & McFarlin, D. E. in Chem. Immun. 46, 101–125 (1989).
Diebler, G. E., Martenson, R. E. & Kies, M. W. Prep. Biochem. 2, 139–165 (1972).
Kent, S. & Clark-Lewis, I. in Synthetic Peptides in Biology and Medicine (eds Alitalo, K., Partonen, P. & Vahen, A.) 29–42 (Elsevier, Amsterdam, 1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lehmann, P., Forsthuber, T., Miller, A. et al. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358, 155–157 (1992). https://doi.org/10.1038/358155a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358155a0
This article is cited by
-
Incorporating the Molecular Mimicry of Environmental Antigens into the Causality of Autoimmune Hepatitis
Digestive Diseases and Sciences (2023)
-
Rotavirus vaccination is not associated with incident celiac disease or autoimmune thyroid disease in a national cohort of privately insured children
Scientific Reports (2022)
-
Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature
Journal of Neurology (2022)
-
Antigen presentation, autoantibody production, and therapeutic targets in autoimmune liver disease
Cellular & Molecular Immunology (2021)
-
Multiple antigen-engineered DC vaccines with or without IFNα to promote antitumor immunity in melanoma
Journal for ImmunoTherapy of Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.