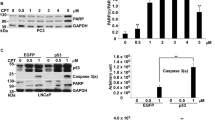
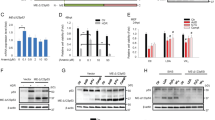
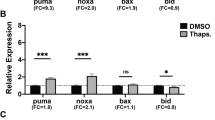
Abstract
Reactive oxygen species have damaging effects on cellular components and so trigger defensive responses by the cell1,2 and even programmed cell death3,4, although the mechanisms by which mammalian cells transmit signals in response to oxidative damage are unknown. We report here that the protein p85, a regulator of the signalling protein phosphatidyl-3-OH kinase (PI(3)K), participates in the cell death process that is induced in response to oxidative stress and that this role of p85 in apoptosis does not involve PI(3)K. We show that disruption of p85 by homologous recombination impairs the cellular apoptotic response to oxidative stress. Because the protein p53 is required for cell death induced by oxidative damage, we examined the relation between p85 and p53. Using a chimaeric p53 fusion protein with the oestrogen receptor (p53ER) to supply p53 (p53 is induced upon binding of p53ER to oestradiol) in a p53-deficient cell line, we found that p85 is upregulated by p53 and that its involvement in p53-mediated apoptosis is independent of PI(3)K. We propose that p85 acts as a signal transducer in the cellular response to oxidative stress, mediating cell death regulated by p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bagchi, D., Bagchi, M., Hassoun, E. A. & Stohs, S. J. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104, 129–140 (1995).
Storz, G., Tartaglia, L. A., Farr, S. B. & Ames, B. N. Bacterial defences against oxidative stress. Trends Genet. 6, 363–368 (1990).
Hockenbery, D. M., Oltvai, Z. N., Yin, X. M., Milliman, C. L. & Korsmeyer, S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75, 241–251 (1993).
Korsmeyer, S. J., Yin, X. M., Oltvai, Z. N., Veis Novack, D. J. & Linette, G. P. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim. Biophys. Acta 1271, 63–66 (1995).
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Hu, P. et al. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell. Biol. 12, 981–990 (1992).
Carpenter, C. L. & Cantley, L. C. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8, 153–158 (1996).
Pleiman, C. M., Hertz, W. M. & Cambier, J. C. Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science 263, 1609–1612 (1994).
Arcaro, A. & Wymann, M. P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 296, 297–301 (1993).
Ferby, I. M., Waga, I., Hoshino, M., Kume, K. & Shimizu, T. Wortmannin inhibits mitogen-activated protein kinase activation by platelet-activating factor through a mechanism independent of p85/p110-type phosphatidylinositol 3-kinase. J. Biol. Chem. 271, 11684–11688 (1996).
Baker, S. J., Markowitz, S., Fearon, E. R., Willson, J. K. & Vogelstein, B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249, 912–915 (1990).
Kern, S. E. et al. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256, 827–830 (1992).
Otsu, M. et al. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell 65, 91–104 (1991).
Kotani, K., Hara, K., Kotani, K., Yonezawa, K. & Kasuga, M. Phosphoinositide 3-kinase as an upstream regulator of the small GTP-bindign protein Rac in the insulin signaling of membrane ruffling. Biochem. Biophys. Res. Commun. 208, 985–990 (1995).
Kapeller, R. & Cantley, L. C. Phosphatidylinositol 3-kinase. Bioessays 16, 565–576 (1994).
Backer, J. M. et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 11, 3469–3479 (1992).
Carpenter, C. L. & Cantley, L. C. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim. Biophys. Acta 1288, 11–16 (1996).
Yao, R. & Cooper, G. M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267, 2003–2006 (1995).
Kauffmann-Zeh, A. et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385, 544–548 (1997).
Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W. & Vogelstein, B. Amodel for p53-induced apoptosis. Nature 389, 300–305 (1997).
Tamemoto, H. et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372, 182–186 (1994).
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual2nd edn (Cold Spring Harbor Laboratory Press, NY, (1989)).
Yin, Y., Tainsky, M. A., Bischoff, F. Z., Strong, L. C. & Wahl, G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell 70, 937–948 (1992).
Preston, G. A. et al. Induction of apoptosis by c-Fos protein. Mol. Cell. Biol. 16, 211–218 (1996).
Hartley, D., Meisner, H. & Corvera, S. Specific association of the beta isoform of the p85 subunit of phosphatidylinositol-3 kinase with the proto-oncogene c-cbl. J. Biol. Chem. 270, 18260–18263 (1995).
Acknowledgements
We thank B. Vogelstein for the plasmid PG13CAT; D. Carbone for H1299 cell line; A.Bradley for p53 MEFs; L. T. Williams and E. Y. Skolnik for p85 cDNAs; G. M. Wahl and T. A. Van Dyke for input and advice; G. A. Preston for DNA ladder analysis; J. E. Stenger for critical comments on themanuscript; and S. Sandberg for help with the manuscript. Y. Yin is grateful to T. D. Tisty and S. T. Lord for their support. This work was supported in part by a Grant-in-Aid for the second term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan (to Y.Y.). Y. Yin was supported by an NIH training fellowship at the University of North Carolina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, Y., Terauchi, Y., Solomon, G. et al. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature 391, 707–710 (1998). https://doi.org/10.1038/35648
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35648
This article is cited by
-
AG1031 induces apoptosis through suppressing SIRT1/p53 pathway in human neuroblastoma cells
Molecular and Cellular Biochemistry (2019)
-
Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury
Acta Pharmacologica Sinica (2017)
-
Fortilin potentiates the peroxidase activity of Peroxiredoxin-1 and protects against alcohol-induced liver damage in mice
Scientific Reports (2016)
-
PTEN regulates RPA1 and protects DNA replication forks
Cell Research (2015)
-
Regulation of p53 by ING family members in suppression of tumor initiation and progression
Cancer and Metastasis Reviews (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.