Abstract
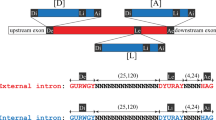
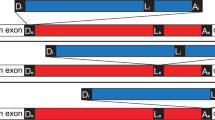
GROUP I and II introns are mobile elements that propagate by insertion into different genes1. Some introns of both types self-splice in vitro by transesterification reactions catalysed by the intron RNA2. These transesterifications are reversible3–5, and it has been suggested that reverse splicing followed by reverse transcription and recombination with genomic DNA may be a mechanism for intron transposition6,7. In vivo the splicing of many, if not all, group I and II introns requires protein factors, which may facilitate correct folding of the intron RNAs8. Here we show that the Neurospora mitochondrial large rRNA intron, a group I intron that is not self-splicing in vitro9, undergoes reverse splicing in a reaction promoted by the CYT-18 protein, the Neurospora mitochondrial tyrosyl-tRNA synthetase, which is required for splicing the intron in vivo10. In contrast to known RNA-catalysed reverse splicing reactions, this protein-assisted reverse splicing is sufficiently rapid to compete with forward splicing at low RNA concentrations under physiologically relevant conditions, including high GTP and low Mg2+ concentrations. Our results indicate that proteins that promote splicing could contribute to intron mobility by promoting reverse splicing in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lambowitz, A. M. Cell 56, 323–326 (1989).
Cech, T. R. A. Rev. Biochem. 59, 543–568 (1990).
Woodson, S. A. & Cech, T. R. Cell 57, 335–345 (1989).
Augustin, S., Müller, M. W. & Schweyen, R. J. Nature 343, 383–386 (1990).
Mörl, M. & Schmelzer, C. Cell 60, 629–636 (1990).
Cech, T. R. Int. Rev. Cytol. 3, 3–22 (1985).
Sharp, P. A. Cell 42, 397–400 (1985).
Lambowitz, A. M. & Perlman, P. S. Trends Biochem. Sci. 15, 440–444 (1990).
Guo, Q., Akins, R. A., Garriga, G. & Lambowitz, A. M. J. biol. Chem. 266, 1809–1819 (1991).
Akins, R. A. & Lambowitz, A. M. Cell 50, 331–345 (1987).
Garriga, G. & Lambowitz, A. M. Cell 46, 669–680 (1986).
Majumder, A. L. et al. Molec. cell. Biol. 9, 2089–2104 (1989).
Kittle, J. D. Jr., Mohr, G., Gianelos, J. A., Wang, H. & Lambowitz, A. M. Genes Dev. 5, 1009–1021 (1991).
Bartel, D. P., Doudna, J. A., Usman, N. & Szostak, J. W. Molec. cell. Biol. 11, 3390–3394 (1991).
Woodson, S. A. & Cech, T. R. Biochemistry 30, 2042–2050 (1991).
Collins, R. A. & Lambowitz, A. M. J. molec. Biol. 184, 413–428 (1985).
Kuiper, M. T. R. & Lambowitz, A. M. Cell 55, 693–704 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohr, G., Lambowitz, A. Integration of a group I intron into a ribosomal RNA sequence promoted by a tyrosyl-tRNA synthetase. Nature 354, 164–167 (1991). https://doi.org/10.1038/354164a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/354164a0
This article is cited by
-
Bacterial group I introns: mobile RNA catalysts
Mobile DNA (2014)
-
Retrotransposition of a bacterial group II intron
Nature (2000)
-
Structure and evolution of myxomycete nuclear group I introns: a model for horizontal transfer by intron homing
Current Genetics (1992)
-
Experimental studies on the origin of the genetic code and the process of protein synthesis: A review update
Origins of life and evolution of the biosphere (1992)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.