Abstract
Zeolites and related crystalline microporous oxides—tetrahedrally coordinated atoms covalently linked into a porous framework—are of interest for applications ranging from catalysis to adsorption and ion-exchange1. In some of these materials (such as zeolite rho) adsorbates2, ion-exchange, and dehydration and cation relocation3,4 can induce strong framework deformations. Similar framework flexibility has to date not been seen in mixed octahedral/tetrahedral microporous framework materials, a newer and rapidly expanding class of molecular sieves5,6,7,8,9,10,11,12,13,14,15,16. Here we show that the framework of the titanium silicate ETS-4, the first member of this class of materials8, can be systematically contracted through dehydration at elevated temperatures to ‘tune’ the effective size of the pores giving access to the interior of the crystal. We show that this so-called ‘molecular gate’ effect can be used to tailor the adsorption properties of the materials to give size-selective adsorbents17 suitable for commercially important separations of gas mixtures of molecules with similar size in the 4.0 to 3.0 Å range, such as that of N2/CH4, Ar/O2 and N2/O2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hammonds, K. D., Heine, V. & Dove, M. T. Rigid-unit modes and the quantitative determination of the flexibility possessed by zeolite frameworks. J. Phys. Chem. B 102, 1759–1767 (1998).
Mentzen, B. F. & Gelin, P. The silicalite p-xylene system. 1. Flexibility of the MFI framework and sorption mechanism observed during p-xylene pore-filling by X-ray powder diffraction at room temperature. Mater. Res. Bull. 30, 373–380 (1995).
Nenoff, T. M. et al. Flexibility of the zeolite RHO framework. In situ X-ray and neutron powder structural characterization of cation exchanged BePO and BeAsO RHO analogs. J. Phys. Chem. 102, 14256–14264 (1996).
Johnson, G. M. et al. Flexibility and cation distribution upon lithium exchange of aluminosilicate and aluminogermanate materials with RHO topologies. Chem. Mater. 11, 2780–2787 (1999).
Kuznicki, S. M. Large-pored crystalline titanium molecular sieve zeolites. US Patent No. 4853202 (1989).
Kuznicki, S. M. & Thrush, K. A. Large-pored molecular sieves with charged octahedral titanium and charged tetrahedral aluminum sites. US Patent No. 5244650 (1993).
Kuznicki, S. M. & Thrush, K. A. Large-pored molecular sieves containing at least one octahedral site comprising titanium and at least silicon as a tetrahedral site. US Patent No. 5208006 (1993).
Kuznicki, S. M. Preparation of small-pored crystalline titanium molecular sieve zeolites. US Patent No. 4938939 (1990).
Anderson, M. W. et al. Structure of the microporous titanosilicate ETS-10. Nature 367, 347–351 (1994).
Anderson, M. W. et al. Microporous titanosilicate ETS-10, a structural survey. Phil. Mag. B 71, 813–841 (1995).
Wang, X. & Jacobson, A. J. Crystal structure of the microporous titanosilicate ETS-10 refined from single crystal X-ray diffraction data. Chem. Commun. 973–974 (1999).
Anderson, M. W. et al. Isomorphous substitution in the microporous titanosilicate ETS-10. Microporous Mater. 6, 195–204 (1996).
Eldewik, A., Luca, V., Singh, N. K. & Howe, R. F. Iron substitution in the microporous titanosilicate ETS-10. Proc. Int. Zeolite Conf. 3, 1507–1514 (1998).
Lamberti, C. Electron-hole reduced effective mass in monoatomic –OTiOTiO– quantum wires embedded in the siliceous crystalline matrix of ETS-10. Microporous Mesoporous Mater. 30, 155–163 (1999).
Rocha, J. & Anderson, M. W. Microporous titanosilicates and other novel mixed octahedral-tetrahedral framework oxides. Eur. J. Inorg. Chem. 801–818 (2000).
Bordiga, S. et al. Stoichiometric and sodium-doped titanium silicate molecular sieve containing atomically defined -OTiOTiO- chains: Quantum ab initio calculations, spectroscopic properties, and reactivity. J. Chem. Phys. 112, 3859–3867 (2000).
Kuznicki, S. M., Bell, V. A., Petrovic, I. & Desai, B. T. Small-pored crystalline titanium molecular sieve zeolites and their use in gas separation processes. US Patent No. 6068682 (2000).
Sandomirskii, P. A. & Belov, N. V. The OD structure of zorite. Sov. Phys. Crystallogr. 24, 686–693 (1979).
Mer'kov, A. N. et al. Raite and zorite, new minerals from the Lovozero tundra. Zap. Vses. Mineral. Obshchest. 102, 54–62 (1973).
Philippou, A. & Anderson, M. W. Structural investigation of ETS-4. Zeolites 16, 98–107 (1996).
Braunbarth, C. et al. Structure of strontium ion exchanged ETS-4 microporous molecular sieves. Chem. Mater. 12, 1857–1865 (2000).
McCusker, L. B. in Comprehensive Supramolecular Chemistry, Solid-State Supramolecular Chemistry: Two- and Three- Dimensional Inorganic Networks (eds Alberti, G. & Bein, T.) 393–423 (Pergamon, New York, 1996).
Cruciani, G., De Luca, P., Nastro, A. & Pattison, P. Rietveld refinement of the zorite structure of ETS-4 molecular sieves. Microporous Mesoporous Mater. 21, 143–153 (1998).
Bieniok, A. & Hammonds, K. D. Rigid unit modes and the phase transition and structural distortions of zeolite rho. Microporous Mesoporous Mater. 25, 193–200 (1998).
Bieniok, A. & Baur, W. H. A large volume contraction accompanies the low- to high- temperature transition of zeolite Sr-RHO. J. Solid State Chem. 90, 173–182 (1991).
Depmeier, W. in Molecular Sieves Vol. 2, Structures and Structure Determination (eds Karge, H. J. & Weitkamp, J.) 113–140 (Springer, Berlin, 1999).
Le Bail, A., Duroy, H. & Fourquet, J. L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 23, 447–452 (1988).
Young, R. A. (ed.) The Rietveld Method (Oxford Univ. Press, Oxford, 1993).
Larson, A. C. & von Dreele, R. B. GSAS Report LAUR 86-784 (Los Alamos National Laboratory, 1986).
Breck, D. W. Zeolite Molecular Sieves (Wiley, New York, 1974).
Mitariten, M. & Dolan, W. Nitrogen removal from natural gas with molecular gate technology. Proc. Laurance Reid Gas Cond. Conf. 51, 1–16 (2001).
Abrams, L. & Corbin, D. R. in Topics in Inclusion Science Vol. 6, Inclusion Chemistry with Zeolites: Nanoscale Materials by Design (eds Herron, N. & Corbin, D. R.) 4–6 (Kluwer, Dordrecht, 1995).
Acknowledgements
We thank J. Curran for discussions and assistance in the preparation of the manuscript, and VTI Corp. for the data of Fig. 5. This work was supported by ATP/NIST, the David and Lucile Packard Foundation and NSF-CTS.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Figures

Figure 1a
(GIF 18.5 KB)
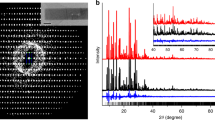
Rietveld refinement of Sr-ETS-4 thermally treated at 150°C showing observed, calculated and difference plots from neutron diffraction data. Tick marks indicate possible Cmmm reflections.

Figure 1b
(GIF 18.1 KB)
Rietveld refinement of Sr-ETS-4 thermally treated at 200°C showing observed, calculated and difference plots from neutron diffraction data. Tick marks indicate possible Cmmm reflections.

Figure 1c
(GIF 18.7 KB)
Rietveld refinement of Sr-ETS-4 thermally treated at 250°C showing observed, calculated and difference plots from neutron diffraction data. Tick marks indicate possible Cmmm reflections.

Figure 1d
(GIF 26.3 KB)
Rietveld refinement of Sr-ETS-4 thermally treated at 300°C showing observed, calculated and difference plots from neutron diffraction data. Tick marks indicate possible Cmmm reflections.

Figure 2
(GIF 23.7 KB)
Powder X-ray diffraction data showing the stability of framework contraction. (a) Room-temperature data before thermal treatment. (b) Two overlaying traces are shown for samples after thermal treatment at 330°C. One is immediately after thermal treatment and the overlay is after 12 days of exposure to humid room-temperature air. After 12 days only a small shift to smaller angles (larger d-spacing) is observed.
Tables
Rights and permissions
About this article
Cite this article
Kuznicki, S., Bell, V., Nair, S. et al. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules. Nature 412, 720–724 (2001). https://doi.org/10.1038/35089052
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35089052
This article is cited by
-
Precise determination of molecular adsorption geometries by room temperature non-contact atomic force microscopy
Communications Chemistry (2024)
-
Simultaneous Thermogravimetry-Differential Scanning Calorimetry (TG-DSC) in Nanoporous Materials: Examples of Data for Zeolites, Metal–Organic Frameworks (MOFs), Clay Based and Mesostructured Solids
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Regulating adsorption performance of zeolites by pre-activation in electric fields
Nature Communications (2023)
-
ZIF-62 glass foam self-supported membranes to address CH4/N2 separations
Nature Materials (2023)
-
Optimization of preparation of NaA zeolite from fly ash for CO2 capture
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.