Abstract
Erythropoietin, a kidney cytokine regulating haematopoiesis (the production of blood cells), is also produced in the brain after oxidative or nitrosative stress1,2. The transcription factor hypoxia-inducible factor-1 (HIF-1) upregulates EPO following hypoxic stimuli3,4. Here we show that preconditioning with EPO protects neurons in models of ischaemic and degenerative damage due to excitotoxins4,5 and consequent generation of free radicals, including nitric oxide (NO). Activation of neuronal EPO receptors (EPORs) prevents apoptosis induced by NMDA (N-methyl-d-aspartate) or NO by triggering cross-talk between the signalling pathways of Janus kinase-2 (Jak2) and nuclear factor-κB (NF-κB). We show that EPOR-mediated activation of Jak2 leads to phosphorylation of the inhibitor of NF-κB (IκB), subsequent nuclear translocation of the transcription factor NF-κB, and NF-κB-dependent transcription of neuroprotective genes. Transfection of cerebrocortical neurons with a dominant interfering form of Jak2 or an IκBα super-repressor blocks EPO-mediated prevention of neuronal apoptosis. Thus neuronal EPORs activate a neuroprotective pathway that is distinct from previously well characterized Jak and NF-κB functions. Moreover, this EPO effect may underlie neuroprotection mediated by hypoxic–ischaemic preconditioning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Digicaylioglu, M. et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc. Natl Acad. Sci. USA 92, 3717–3720 (1995).
Masuda, S. et al. Functional erythropoietin receptors of the cells with neuronal characteristics—comparison with receptor properties from erythroid cells. J. Biol. Chem. 268, 11208–11216 (1993).
Bernaudin, M. et al. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia 30, 271–278 (2000).
Morishita, E., Masuda, S., Nagao, M. & Sasaki, R. Erythropoietin receptor is expressed in rat hippocampal cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 76, 105–116 (1997).
Sirén, A.-L. et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl Acad. Sci. USA 98, 4044–4049 (2001).
Anagnostou, A., Lee, E. S., Kessimian, N., Levinson, R. & Steiner, M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl Acad. Sci. USA 87, 5978–5982 (1990).
Parganas, E. et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 (1998).
Lipton, S. A. et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364, 626–632 (1993).
Dawson, V. L., Dawson, T. M., Bartley, D. A., Uhl, G. R. & Snyder, S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J. Neurosci. 13, 2651–2661 (1993).
Bonfoco, E., Krainc, D., Ankarcrona, M., Nicotera, P. & Lipton, S. A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl Acad. Sci. USA 92, 7162–7166 (1995).
Gregory, T. et al. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood 94, 87–96 (1999).
Bonfoco, E. et al. Bcl-2 delays apoptosis and PARP cleavage induced by NO donors in GT1-7 cells. Neuroreport 8, 273–276 (1996).
Beg, A. A. & Baltimore, D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274, 782–784 (1996).
Wang, C. Y., Mayo, M. W. & Baldwin, A. S. Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274, 784–787 (1996).
Van Antwerp, D. J., Martin, S. J., Kafri, T., Green, D. R. & Verma, I. M. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274, 787–789 (1996).
Grilli, M., Pizzi, M., Memo, M. & Spano, P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science 274, 1383–1385 (1996).
Wang, C. Y., Mayo, M. W., Korneluk, R. G., Goeddel, D. V. & Baldwin, A. S. Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680–1683 (1998).
O'Neill, L. A. & Kaltschmidt, C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20, 252–258 (1997).
Mattson, M. P., Goodman, Y., Luo, H., Fu, W. & Furukawa, K. Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res. 49, 681–697 (1997).
Schreck, R., Meier, B., Mannel, D. N., Droge, W. & Baeuerle, P. A. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J. Exp. Med. 175, 1181–1194 (1992).
Lin, Y. Z., Yao, S. Y., Veach, R. A., Torgerson, T. R. & Hawiger, J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270, 14255–14258 (1995).
Iimuro, Y. et al. NF-κB prevents apoptosis and liver dysfunction during liver regeneration. J. Clin. Invest. 101, 802–811 (1998).
Lezoualc’h, F., Sagara, Y., Holsboer, F. & Behl, C. High constitutive NF-κB activity mediates resistance to oxidative stress in neuronal cells. J. Neurosci. 18, 3224–3232 (1998).
Ihle, J. N., Witthuhn, B. A., Quelle, F. W., Yamamoto, K. & Silvennoinen, O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 13, 369–398 (1995).
Meydan, N. et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379, 645–648 (1996).
Briscoe, J. et al. Kinase-negative mutants of JAK1 can sustain interferon-gamma-inducible gene expression but not an antiviral state. EMBO J. 15, 799–809 (1996).
Zhuang, H. et al. Inhibition of erythropoietin-induced mitogenesis by a kinase-deficient form of Jak2. J. Biol. Chem. 269, 21411–21414 (1994).
Gage, A. T. & Stanton, P. K. Hypoxia triggers neuroprotective alterations in hippocampal gene expression via a heme-containing sensor. Brain Res. 719, 172–178 (1996).
Schmidt, H. H. H. W. & Kelm, M. in Methods in Nitric Oxide Research (eds Feelisch, M. & Stamler, J. S.) 491–497 (Wiley, Chichester, 1996).
Imbert, V. et al. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell 86, 787–798 (1996).
Acknowledgements
We thank M. Kaul, N. Moayeri, B. Price, M. Cokol and M. Altinoz for insightful discussions or technical advice, and the Genetics Institute, Cambridge, Massachusetts, for supplying the anti-EPOR monoclonal antibodies. The complementary DNA strands for the IκB super-repressor (Ad5IκB) and kinase-negative mutant Jak2 (JAK2.KE) were the gifts of R. R. Ratan and J. Ihle, respectively. This work was supported in part by grants from the National Institutes of Health and American Heart Association (S.A.L.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
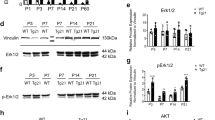
We characterized the expression of EPO receptor (EPO-R) protein on rat brain neurons by immunohistochemistry of brain sections with a specific monoclonal antibody (Fig. 1a, left panel). To perform the studies elucidating the signalling pathway that mediates EPO neuroprotection, we sought evidence for EPO-Rs on cultured rat cerebrocortical neurons using immunofluorescence and immunoblotting with the same specific anti-EPO-R mAb that we used in vivo6. Double-labeling of cerebrocortical neurons with anti-EPO-R mAb and an antibody against neuron-specific microtubule-associated protein-2 (MAP-2), revealed complete overlap of the two labels (WebFig. 1a, right panel). This anti-EPO-R mAb recognized a single band in immunoblots prepared from soluble EPO-R (Fig. 1b, right) and from lysates of the erythroid cell line UT-7 and HEK293, both of which express EPO-R, but not from lysates of HeLa cells, which do not express the EPO-R (WebFig. 1b, lower left). Anti-EPO-R also recognized a single band of appropriate size in immunoblotted cell lysates prepared from cerebrocortical cultures containing neurons but not from neuron-depleted cultures (WebFig. 1b, upper left). In neuronal cell lysates, immunoprecipitated EPO-R and Jak2 were tyrosine phosphorylated by EPO (Fig. 1c).
Hitherto, it was known that activation of several cytokine receptors, of which the EPO-R is a member, could trigger the Janus kinase-signal transducers and activators of transcription (Jak-Stat) signalling pathway. It was also known that phosphorylated Stats then translocate into the nucleus to act as transcription factors. Based upon results in the present study, we propose that binding of EPO to EPO-R activates a novel form of cross-talk between the well-known Jak2 and NF-kB pathways, in which Jak2 activation leads to IkB phosphorylation. IkB is then either degraded (after serine phosphorylation) or dissociates without degradation (in the case of tyrosine phosphorylation) from NF-kB, which subsequently translocates into the nucleus to act as a transcription factor (WebFig. 2). In this manner, NF-kB participates in EPO-mediated neuroprotection. This EPO signalling cascade may prevent neuronal apoptosis after cerebral infarcts and other neurodegenerative insults involving excessive NMDA receptor activation and NO generation, and thus may have potential therapeutic value.
Methods
Immunofluorescence and immunoblot studies
Frozen sections (20 µm) of rat hippocampus were labelled with anti-EPO-R mAb (Genetics Institute), visualized with horseradish peroxidase (HRP)/diaminobenzidine (DAB), and counterstained with Giemsa. For cultured cerebrocortical cells, neurons were double labelled with anti-EPO-R and the neuronal markers anti-MAP-2 or anti-NeuN. FITC-conjugated secondary antibody was used to visualize the EPO-R, and Texas-Red-conjugated secondary antibody was used for MAP-2 or NeuN. For localization of NF-kB and Jak2, cells were fixed in 4% paraformaldehyde, incubated overnight with anti-NF-kB (p65 subunit) or anti-Jak2 polyclonal antibodies (Santa Cruz Biotechnology), and visualized with FITC conjugated secondary antibody. Neurons were identified with anti-MAP-2 or anti-NeuN, and astrocytes with anti-glial fibrillary acidic protein (GFAP); these markers were visualized with secondary antibodies conjugated to Texas Red or HRP developed with DAB and H2O2. Incubation in 200 µM NMDA overnight yielded neuron-depleted cultures10. Soluble EPO-R protein was resolved on SDS-PAGE and labelled on immunoblots with anti-EPO-R mAb.
Supplementary Figures

Figure 1
(JPG 56.4 KB)
Erythropoietin receptors (EPO-Rs) on rat primary cerebrocortical neurons. a, EPO-Rs in the hippocampus of embryonal rat brain (E19) (arrows). Frozen sections labeled with monoclonal anti-EPO-R (left panel, 400x). Cultured cerebrocortical neurons double labelled with anti-EPO-R (green) and neuronal marker MAP-2 (red; right panel, 630x). b, On western blots with anti-EPO-R mAb, EPO-R protein was virtually absent in neuron-depleted cultures (labelled "—") but detectable in cultures with neurons (+; upper left panel). Anti-EPO-R recognized EPO-R protein expressed in the EPO-dependent cell line UT-7 and in HEK 293 cells, but not in HeLa cells, which are known to lack EPO-Rs (bottom left panel). Increasing amounts of soluble EPO-R protein (sEPO-R) were recognized on western blots with anti-EPO-R mAb (right panel). c, EPO incubation induced tyrosine phosphorylation of EPO-R and Jak2 proteins. Cell lysates of cerebrocortical cultures incubated with increasing concentrations of EPO were immunoprecipitated with anti-EPO-R and immunoblotted with anti-phosphotyrosine antibody (p-Tyr; left panel). The same immunoblot was then stripped and reprobed with the anti-EPO-R or anti-Jak2 (right panel).

Figure 2
(JPG 40.8 KB)
Schematic model of EPO-induced NF-kB activation for neuroprotection. EPO binding to the EPO-R induces receptor dimerization, followed by activation of the tyrosine kinase Jak2. In nonneuronal cells, EPO-induced Jak2 kinase activity is known to phosphorylate/activate Stat5. Here we show in neuronal cells that activated Jak2 may directly or indirectly lead to phosphorylation of IkB at tyrosine or serine residues, respectively. Serine phosphorylation may possibly be mediated via IKK complex and is the predominant pathway for NF-kB activation. Jak2-mediated phosphorylation of IkB represents a novel form of cross-talk between the Jak-Stat and NF-kB signalling pathways. Phosphorylation of IkB results in its degradation (after serine phosphorylation) or non-degradory dissociation (after tyrosine phosphorylation) from NF-kB, which then translocates into the nucleus to transcriptionally activate antiapoptotic genes (box at right; SOD, superoxide dismutase).
Rights and permissions
About this article
Cite this article
Digicaylioglu, M., Lipton, S. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature 412, 641–647 (2001). https://doi.org/10.1038/35088074
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35088074
This article is cited by
-
Neuroprotective therapies in the NICU in preterm infants: present and future (Neonatal Neurocritical Care Series)
Pediatric Research (2023)
-
Erythropoietin regulates signaling pathways associated with neuroprotective events
Experimental Brain Research (2022)
-
Recombinant human erythropoietin and interferon-β-1b protect against 3-nitropropionic acid-induced neurotoxicity in rats: possible role of JAK/STAT signaling pathway
Inflammopharmacology (2022)
-
Interleukin-17A up-regulates thymic stromal lymphopoietin production by nasal fibroblasts from patients with allergic rhinitis
European Archives of Oto-Rhino-Laryngology (2021)
-
Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.